Synthesis of cyclic metal iridium coordination compound and application of cyclic metal iridium coordination compound in preparing polymer white-light devices
A technology of iridium complexes and white light devices, which is applied in the field of preparation of polymer white light devices, can solve the problems of large energy consumption, environmental damage, low energy utilization rate, etc., and achieve improved luminous performance, high efficiency, increased hole injection and The effect of transmission capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of 4-(5-(9,9-diethylfluoren-2yl)pyridin-2-yl)-N,N-diphenylaniline
[0035]
[0036] (1) Synthesis of 4-(5-bromopyridin-2-yl)-N,N-diphenylaniline
[0037] 3.71g (10.0mmol) N,N-diphenyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline, 2.37g (10.0mmol) 2,5-dibromopyridine and 20mL 2.0M potassium carbonate aqueous solution, and 40mL toluene and 20mL ethanol were added to a 150mL two-necked flask, and 60mg (0.05mmol) tetrakis (triphenylphosphine) palladium was added under argon protection , heated to 90°C, and reacted for 24h. After cooling to room temperature, the reaction solution was poured into deionized water, extracted three times with dichloromethane, and the organic phases were combined and washed with water. Anhydrous MgSO 4 After drying overnight, the volatile solvent was evaporated by a rotary evaporator. The crude product was separated and purified by chromatographic column (silica gel as stationary phase, petroleum ether / dichloromethane =...
Embodiment 2
[0041] Synthesis of 4-(6-(9,9-diethylfluoren-2-yl)pyridin-3-yl)-N,N-diphenylaniline
[0042]
[0043] (1) Synthesis of 5-bromo-2-(9,9-diethylfluoren-2-yl)pyridine
[0044] 5.16g (10.0mmol) 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborinane)-9,9-diethylfluorene, 2.37g (10.0mmol) 2,5-dibromopyridine and 20mL 2.0M potassium carbonate aqueous solution, and 40mL toluene and 20mL ethanol were added to a 150mL two-necked flask, and 60mg (0.05mmol) tetrakis (triphenylphosphine) palladium was added under the protection of argon, and heated to 90 °C, react for 24h. After cooling to room temperature, the reaction solution was poured into deionized water, extracted three times with dichloromethane, and the organic phases were combined and washed with water. Anhydrous MgSO 4 After drying overnight, the volatile solvent was evaporated by a rotary evaporator. The crude product was separated and purified by chromatographic column (silica gel as stationary phase, petroleum ether / dichloromethane...
Embodiment 3
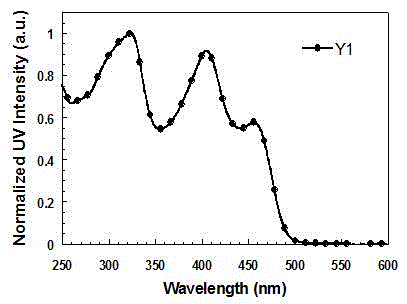
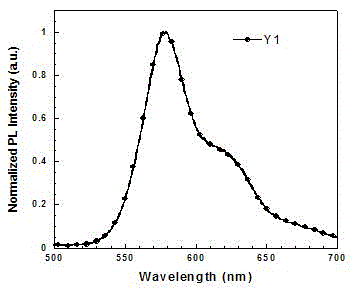
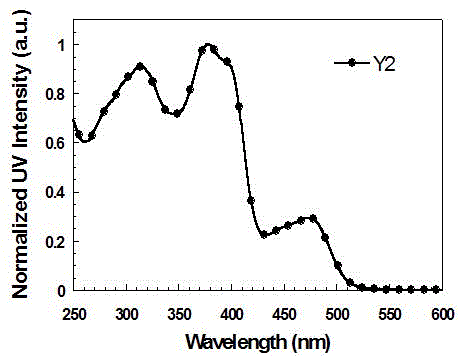
[0048] Synthesis of Functionalized Cyclometallic Iridium Complexes Y1 and Y2
[0049]
[0050] (1) Bis(4-(5-(9,9-diethylfluoren-2-yl)pyridin-2-yl)-N,N-diphenylaniline-N,C 2 ) (picolinic acid) iridium (Ⅲ) is the synthesis of Y1
[0051] Add 2.1 g (3 mmol) of 4-(5-(9,9-diethylfluoren-2-yl)pyridin-2-yl)-N,N-diphenylaniline, 21 mL of ethylene glycol monoethyl ether and 7 mL of water To a 50mL three-neck flask, quickly add 0.42g (1.2mmol) IrCl under the protection of argon 3 ·3H 2 O, 100 ℃ constant temperature reaction 20h. After cooling, a yellow solid was produced, which was filtered by suction, washed with water and a little ethanol successively, and dried in vacuum to obtain 1.78 g of a yellow powder. The product was directly used in the next reaction without further separation and purification.
[0052] In a 50 mL three-neck flask, add 1.3 g (0.4 mmol) of the reaction product from the previous step, 193 mg (1.6 mmol) of 2-pyridinecarboxylic acid, 424 mg (4.0 mmol) of s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum luminance | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Maximum lumen efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com