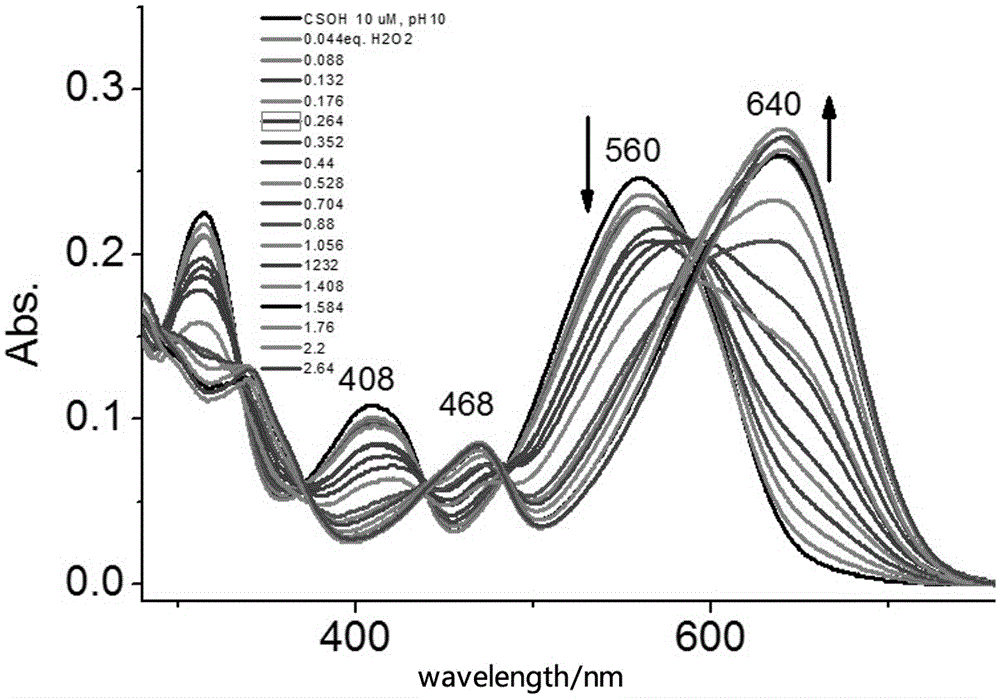
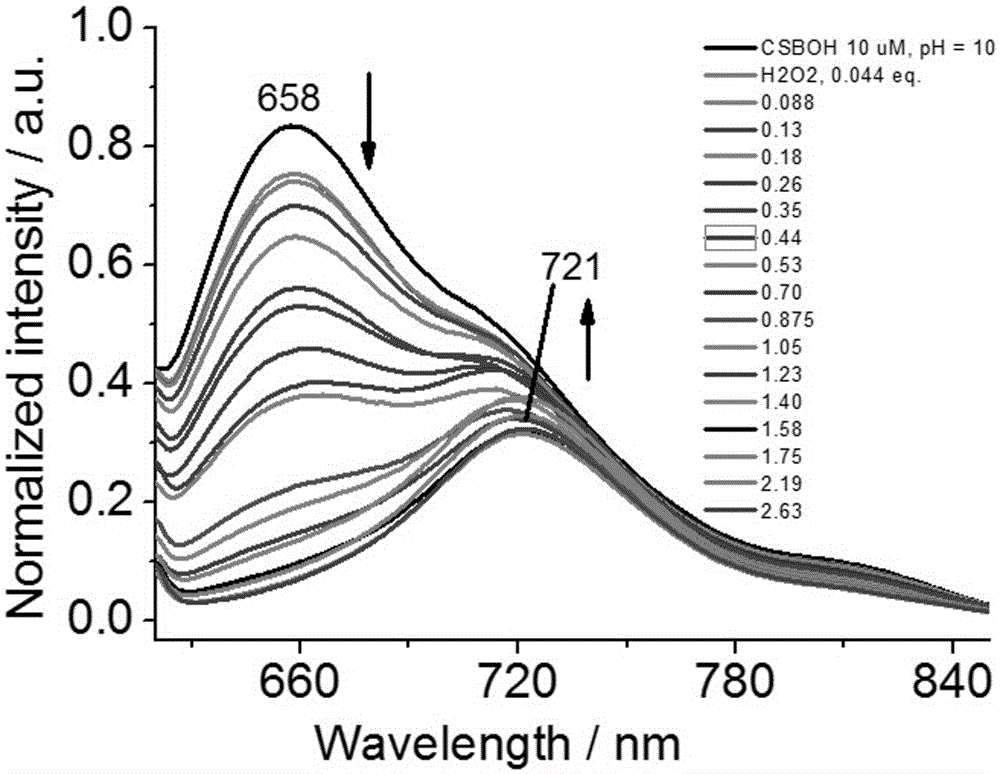
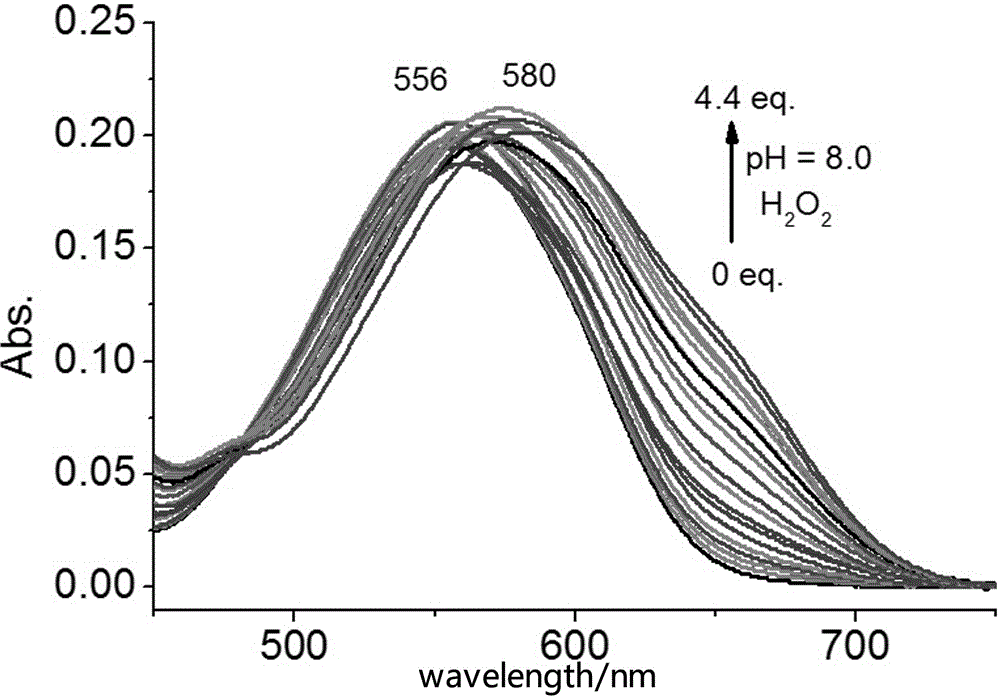
Novel fluorescence probe for detecting hydrogen peroxide in alkaline environment and preparation method and biological application thereof
A fluorescent probe and alkaline environment technology, applied in the field of organic small molecule fluorescent probes, can solve the problems of few types of probes, insufficient sensitivity of detection signals, lack of fluorescent probes, etc., and achieve obvious phenomena, simple preparation methods, and detection highly sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of compound 1
[0039] React m-N-diethylaminophenol (0.50 g, 3.03 mmol) with phthalic anhydride (0.49 g, 3.33 mmol) in 180 mL of benzene at 60°C for 24 hours to obtain a white solid, then add the white solid to 20 mL of 98 wt% concentrated sulfuric acid containing 6 ml of cyclohexanone was reacted at 50°C for 3 hours, and the solid was recrystallized from ethanol to obtain compound 1 (0.22 g, 0.45 mmol).
[0040] The synthetic route is as follows:
Embodiment 2
[0042] Preparation of a Novel Fluorescent Probe for Detecting Hydrogen Peroxide in Alkaline Environment
[0043] The reaction scheme is as follows:
[0044]
[0045] The preparation process comprises steps as follows:
[0046] Compound 1 (0.377 g, 1.0 mmol) and p-borate benzaldehyde (0.157 g, 1.2 mmol) were reacted in 20 ml of acetic acid at 50 °C for 12 h to obtain a dark solution, and the solvent was removed by rotary distillation. Chloromethane was dissolved, and the mixed solvent of dichloromethane and methanol was separated by column chromatography to obtain the target compound CS-BOH ; Yield: 50%. 1 H NMR (400 MHz, DMSO-d6) δ 8.16 (m, 2H), 8.06 (s, 1H), 7.86 (t, J = 7.2, 2H), 7.72 (d, J = 8.4 , 1H), 7.51 (s, 2H), 7.35 (d, J = 7.6 , 1H), 6.8 (m, 2H), 3.58 (q, J = 6.4 , 4H ), 2.8 (m, 2H), 2.07 (m, 1H), 1.67 (m, 3H), 1.23 (t, J = 6.8 , 6H ). 13 C NMR (101 MHz, CDCl3) δ 169.94, 152.38, 152.09, 149.39, 146.29, 142.08, 134.56, 133.47, 131.95, 129.98, 129.38, 128....
Embodiment 3
[0048] The preparation method of hydrogen peroxide fluorescent probe as described in embodiment 2, difference is:
[0049] The amount of p-borate benzaldehyde was 3 mmol, acetic acid was replaced by acetic anhydride, the amount of acid was 20 mL, the reaction temperature was 60 °C, and the reaction time was 12 h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com