Compound norfloxacin sustained release tablet and preparation method
A technology of norfloxacin and sustained-release tablets, which is applied in the field of compound norfloxacin sustained-release tablets and its preparation, can solve the problems of rapid-release preparations, such as frequent medication, diarrhea, nausea or vomiting, and poor patient compliance, to achieve enhanced Immunity of the body, improvement of compliance, and maintenance of drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
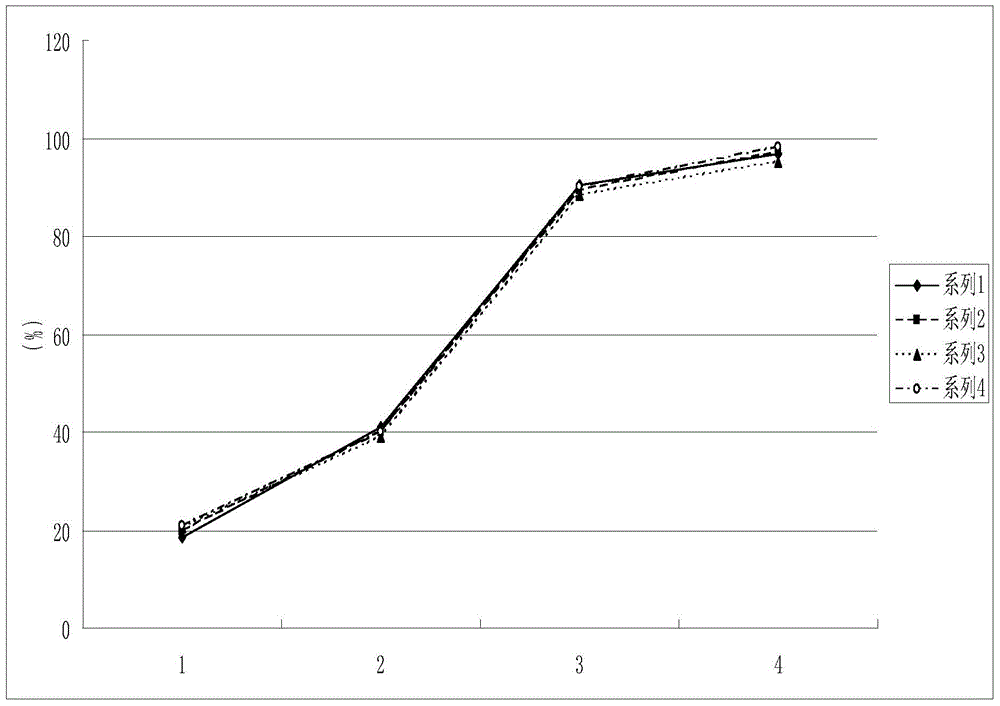
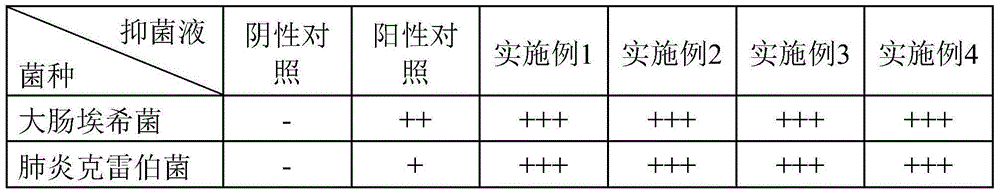
Image
Examples
Embodiment 1
[0026] A compound norfloxacin sustained-release tablet. The components and parts by weight of the sustained-release tablet include 4 parts of norfloxacin, 33 parts of frankincense, 38 parts of Guya, 35 parts of divine music, 27 parts of dried ginger, Lu Ying 12 parts, 18 parts of Atractylodes macrocephala, 13 parts of Morinda officinalis, 35 parts of red dates, 6 parts of Magnolia officinalis leaves, 19 parts of Citrus aurantii, 13 parts of sodium alginate, 8 parts of starch, 0.7 parts of polysorbate, and 0.8 parts of micronized silica gel.
[0027] A preparation method of compound norfloxacin sustained-release tablet, comprising the following steps:
[0028] 1) preparing materials, preparing materials according to the components and parts by weight of a kind of compound norfloxacin sustained-release tablet, wherein the pharmaceutical auxiliary materials are pulverized through 80 mesh sieves, and norfloxacin is pulverized through 120 mesh sieves;
[0029]2) Extraction of the e...
Embodiment 2
[0036] A compound norfloxacin sustained-release tablet, the components and parts by weight of the sustained-release tablet include 3 parts of norfloxacin, 30 parts of frankincense, 35 parts of Guya, 30 parts of divine music, 25 parts of dried ginger, Lu Ying 10 parts, 15 parts of Atractylodes macrocephala, 10 parts of Morinda officinalis, 30 parts of red dates, 5 parts of Magnolia officinalis leaves, 15 parts of Citrus aurantii, 10 parts of sodium alginate, 5 parts of starch, 0.5 parts of polysorbate, and 0.5 parts of micronized silica gel.
[0037] A preparation method of compound norfloxacin sustained-release tablet, comprising the following steps:
[0038] 1) preparing materials, preparing materials according to the components and parts by weight of a kind of compound norfloxacin sustained-release tablet, wherein the pharmaceutical auxiliary materials are pulverized through 80 mesh sieves, and norfloxacin is pulverized through 120 mesh sieves;
[0039] 2) Extraction of the ...
Embodiment 3
[0046] A compound norfloxacin sustained-release tablet. The components and parts by weight of the sustained-release tablet include 5 parts of norfloxacin, 35 parts of frankincense, 40 parts of Guya, 40 parts of divine music, 30 parts of dried ginger, Lu Ying 15 parts, 20 parts of Atractylodes macrocephala, 15 parts of Morinda officinalis, 40 parts of red dates, 10 parts of Magnolia officinalis leaves, 20 parts of Citrus aurantii, 15 parts of sodium alginate, 10 parts of starch, 1 part of polysorbate, and 1 part of micronized silica gel.
[0047] A preparation method of compound norfloxacin sustained-release tablet, comprising the following steps:
[0048] 1) preparing materials, preparing materials according to the components and parts by weight of a kind of compound norfloxacin sustained-release tablet, wherein the pharmaceutical auxiliary materials are pulverized through 80 mesh sieves, and norfloxacin is pulverized through 120 mesh sieves;
[0049] 2) Extraction of the effe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com