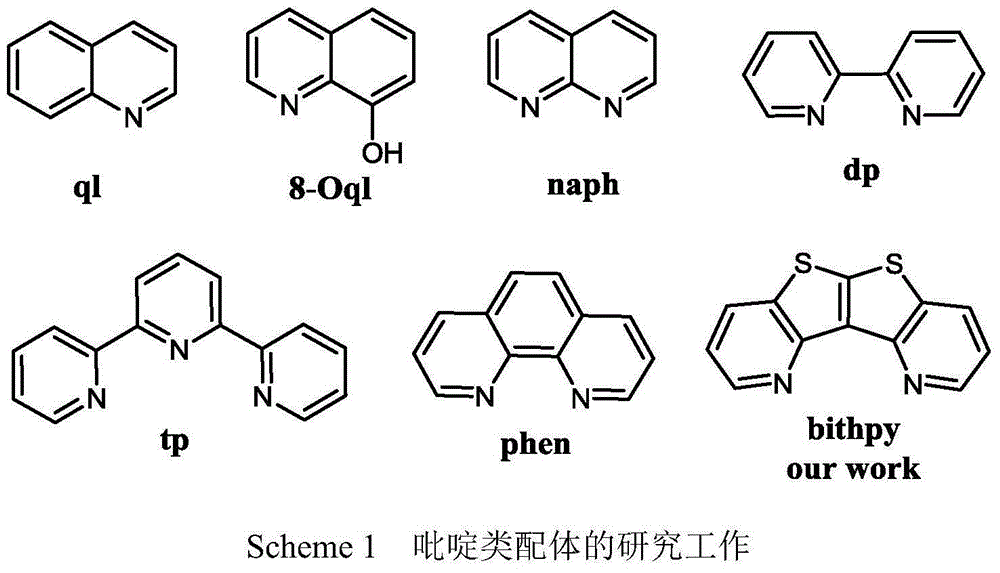
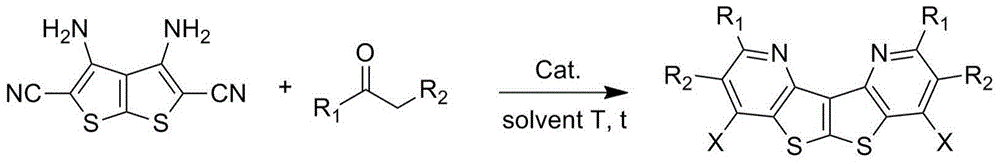
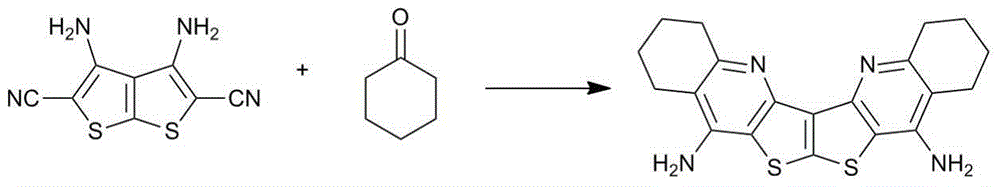
Bi-thienopyridine type derivative and preparation method thereof
A technology of phenopyridines and derivatives is applied in the field of organic synthesis, which can solve the problem of high yield and achieve the effects of high yield, high atom economy and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile (1mmol) and cyclohexanone (3mL) into a 25ml single-necked flask, stir until uniformly dispersed, add AlCl 3 (2mmol), and then rapidly heated to reflux, and reacted with magnetic stirring for 3.0h. After the reaction, directly filter and wash with water to obtain a solid mixture. The crude product was recrystallized from DMF to obtain a white solid (1) with a yield of 74% (the recrystallization mother liquor was recovered). The reaction formula of 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile and cyclohexanone is:
[0030]
[0031] The spectral data of product (1) is: 1 H NMR (DMSO-d 6 ,400MHz)δ:7.25(s,4H,NH 2 ),3.27(s,4H,CH 2 ),2.57(s,4H,CH 2 ), 1.83(s,8H,CH2). 13 C NMR (DMSO-d 6 ,100MHz) δ: 153.8, 149.7, 145.2, 143.9, 118.9, 111.4, 109.2, 31.0, 23.3, 21.76.
Embodiment 2
[0033] Add 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile (1mmol) and cyclopentanone (5mL), 2ml toluene into a 10ml microwave tube, stir until uniformly dispersed, add ZnCl 2 (0.5 mmol), reacted at 120° C. for 30 min with a microwave reaction synthesizer. After the reaction, filter and wash with water. The crude product was recrystallized from ethanol to obtain a light brown solid (2) with a yield of 70% (recovered from the recrystallized mother liquor). The reaction formula of 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile and cyclopentanone is:
[0034]
[0035] The spectral data of product (2) is: 1 H NMR (DMSO-d 6 ,400MHz)δ:7.36(s,4H,NH 2 ),3.20(t,J=7.77Hz,4H,CH 2 ), 2.87(t, J=7.33Hz, 4H, CH 2 ),2.21-2.17(m,4H,CH 2 ). 13 C NMR (DMSO-d 6,100MHz) δ: 162.0, 148.1, 145.0, 144.4, 119.6, 116.4, 32.9, 27.6, 22.0.
Embodiment 3
[0037] In a 25 mL mortar, add 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile (1 mmol) and cycloheptanone (6 mL), 3 mL of methanol, stir until uniformly dispersed, Add p-toluenesulfonic acid (3 mmol) and triturate at room temperature for 30 min. After the reaction, 5 mL of ethanol was added, filtered and washed with water. The crude product was recrystallized from a mixed solvent of methanol and dichloromethane to obtain an off-white solid (3) with a yield of 67% (recovered from the recrystallization mother liquor). The reaction formula of 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile and cycloheptanone is:
[0038]
[0039] The spectral data of product (3) is 1 H NMR (DMSO-d 6 ,400MHz)δ:7.20(s,4H,NH 2 ), 3.35 (d, J=9.25, 4H, CH 2 ), 2.88 (d, J=9.15, 4H, CH 2 ),1.82(s,4H,CH 2 ),1.69(s,4H,CH 2 ), 1.59 (d, J=6.94, 4H, CH 2 ). 13 C NMR (DMSO-d 6 ,100MHz) δ: 160.8, 148.8, 143.1, 120.4, 116.9, 43.2, 36.5, 30.8, 29.8, 26.3, 23.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com