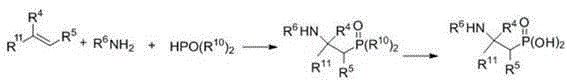
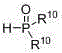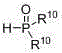Preparation method of beta-aminoethylphosphonyl derivatives
A technology of aminoethylphosphonyl derivatives and ethylene derivatives, which is applied in the field of organic synthesis, can solve the problems of difficulty in obtaining raw materials, rare reaction raw materials, harsh reaction conditions, etc., and achieves reduction of waste generation, reaction operation and post-processing The effect of simple processing and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of 2-amino-2-phenylethylphosphonic acid
[0052] Add methanol (6 mL), styrene (0.104 g, 1 mmol), ammonia (0.12 g, 4.0 mmol), dimethyl phosphinate (0.22 g, 2 mmol), silver nitrate (0.340 g, 2 mmol), copper bromide (0.045 g, 0.2 mmol), the mixture was stirred and reacted at 40 °C, and the reaction was tracked by TLC until the end. After the reaction, half of the reaction solution was taken out, and the crude product obtained after concentration was separated by column chromatography (petroleum ether / acetone / dichloromethane=20 / 1 / 1) to obtain the target product 2-amino-2-phenylethane phosphonate (yield 81%); 1 H NMR (400 MHz, CDCl 3 ): δ 8.50 (s, 2H),7.18-7.42 (m, 5H ), 4.11-4.31 (m, 1H ) , 3.63 (d, J = 10.8 Hz, 6H), 1.60-1.80 (m, 2H ) .
[0053] Add 7.5 mL of 20% hydrochloric acid to the remaining half of the reaction solution in the reaction bottle, heat the mixture to reflux, and follow the reaction by TLC until the end; add an appropriate ...
Embodiment 2
[0054] Example two: Synthesis of 2-amino-2-(4-chlorophenyl)ethylphosphonic acid
[0055] Add ethanol (6 mL), 4-chlorostyrene (0.139 g, 1 mmol), ammonia water (0.12 g, 4.0 mmol), dimethyl phosphinate (0.22 g, 1 mmol), silver nitrate ( 0.340 g, 2 mmol), copper bromide (0.045 g, 0.2 mmol), the mixture was stirred and reacted at 30°C, and the reaction was tracked by TLC until the end. After the reaction, half of the reaction solution was taken out, and the crude product obtained after concentration was separated by column chromatography (petroleum ether / acetone / dichloromethane=20 / 1 / 1) to obtain the target product 2-amino-2-(4- Chlorophenyl) ethylphosphonate (84% yield). Its analysis data is as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.50 (s, 2H), 7.57–7.43 (m, 4H), 4.11-4.31 (m, 1H ) , 3.64 (d, J = 10.8 Hz, 6H), 1.61-1.80 (m, 2H ) .
[0056] Add 7.5 mL of 20% hydrochloric acid to the remaining half of the reaction solution in the reaction bottle, heat the mixture to reflux...
Embodiment 3
[0057] Embodiment three: the synthesis of 2-aminoethylphosphonic acid
[0058] Acetonitrile (6 mL), acrylic acid (0.072 g, 1 mmol), ammonia water (0.24 g, 8.0 mmol), diethyl phosphinate (0.276 g, 2 mmol), silver nitrate (0.340 g, 2 mmol) were added to the reactor. mmol), copper bromide (0.045 g, 0.2 mmol), the mixture was stirred and reacted at 50 °C, and the reaction was tracked by TLC until the end. After the reaction, half of the reaction solution was taken out, and the crude product obtained after concentration was separated by column chromatography (petroleum ether / acetone / methylene chloride=20 / 1 / 1) to obtain the target product 2-aminoethylphosphonate ( Yield 78%). Its analysis data is as follows: 1 H NMR (300 MHz, D 2 O): δ 4.30-4.50 (m, 1H ) , 4.10?3.95 (m, 4H), 1.72-1.93 (m, 2H), 1.27 (t, J = 7.1 Hz, 6H) .
[0059] Add 7.5 mL of 20% hydrochloric acid to the remaining half of the reaction solution in the reaction flask, heat the mixture to reflux, and track the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com