Medicinal erdosteine composition tablet for treating respiratory tract inflammation and preparation method of tablet
A technology of erdosteine and composition, which is applied in the field of erdosteine composition tablet for the treatment of respiratory inflammation, which can solve the problems of drug absorption speed or degree, poor water solubility of erdosteine, and effective bioavailability Low-level problems, to achieve the effect of high stability, good stability and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of Erdosteine Crystals
[0026] Get the erdosteine raw material drug, add in the mixed solvent A of acetone, cyclohexane, N-methylacetamide whose volume is 8 times of the weight of erdosteine at 30 ℃, acetone, cyclohexane, N-methyl Acetamide volume ratio is 3:2:2, obtains solution; Apply the constant magnetic field that magnetic field intensity is 1.5T then in the horizontal direction of the liquid surface of gained solution, and drop volume is in solution under the condition of this constant magnetic field. Mixed solvent B of methanol, isobutanol and water with 10 times the weight of erdosteine, the volume ratio of methanol, isobutanol and water is 2:3:4; hours, filtered, washed, and vacuum-dried to obtain the erdosteine crystals.
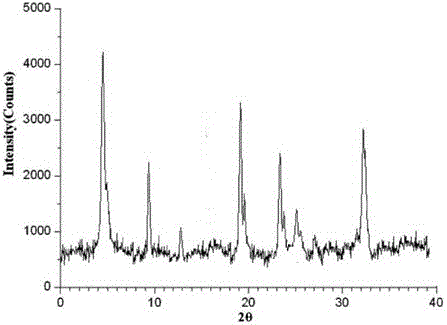
[0027] The X-ray powder diffraction pattern obtained by measuring the obtained erdosteine crystal using Cu-Kα ray is as follows: figure 1 Shown, its purity as determined by high performance liquid chroma...
Embodiment 2
[0028] Example 2: The preparation of erdosteine sheet, step is as follows:
[0029] Prescription: in parts by weight
[0030]
[0031]
[0032] Preparation:
[0033] 1) Processing of raw and auxiliary materials: Pass erdosteine, sodium starch glycolate, lactose, and L-HPC21 through an 80-mesh sieve;
[0034] 2) Weighing: Weighing according to the process prescription;
[0035] 3) Mixing and granulation: Add the prescribed amount of erdosteine, sodium starch glycolate, lactose, and L-HPC21 into the wet mixing granulator, turn on the stirring motor for dry mixing for 10 minutes, add 95% ethanol solution, wet Mix and cut 100-120 seconds to make soft materials, choose 16-mesh nylon mesh and install it in a swinging granulator to granulate;
[0036] 4) Drying: Adjust the inlet air temperature of the boiling dryer to 55-65°C, place the wet granules in the boiling dryer for drying until the moisture content is 1.5%-2.5%;
[0037] 5) Total blending: add the sized dry gra...
Embodiment 3
[0040] Example 3: The preparation of erdosteine sheet, step is as follows:
[0041] Prescription: in parts by weight
[0042]
[0043] Preparation:
[0044] 1) Processing of raw and auxiliary materials: Pass erdosteine, sodium starch glycolate, lactose, and L-HPC21 through an 80-mesh sieve;
[0045] 2) Weighing: Weighing according to the process prescription;
[0046]3) Mixing and granulation: Add the prescribed amount of erdosteine, sodium starch glycolate, lactose, and L-HPC21 into the wet mixing granulator, turn on the stirring motor for dry mixing for 10 minutes, add 95% ethanol solution, wet Mix and cut 100-120 seconds to make soft materials, choose 16-mesh nylon mesh and install it in a swinging granulator to granulate;
[0047] 4) Drying: Adjust the inlet air temperature of the boiling dryer to 55-65°C, place the wet granules in the boiling dryer for drying until the moisture content is 1.5%-2.5%;
[0048] 5) Total blending: add the sized dry granules, the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com