Drug for targeted therapy of Alzheimer's disease (AD) and preparation method thereof
A technology of targeted therapy for Alzheimer's disease, applied in gene therapy, drug combination, pharmaceutical formula, etc., can solve problems such as low efficiency of non-viral carrier interference, safety problems, retinopathy, etc., and achieve the relief of Alzheimer's symptoms, Inhibition of production and deposition, high biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of the magnetic mesoporous silica / SiRNA nanocomposite of embodiment 1.PHI polypeptide modification
[0038] Preparation:
[0039] 1) Synthesis of M-MSNs materials:
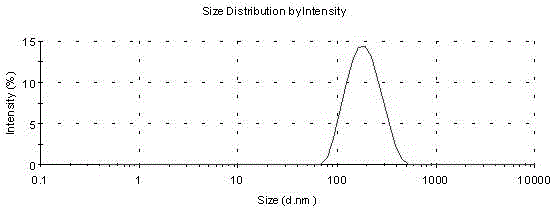
[0040] Synthesis of M-MSNs: Monodisperse Fe 3 o 4 The particles were synthesized by co-precipitation, and the product was stably modified with oleic acid (purchased from Sinopharm Chemical Reagent Co., Ltd.) and then stored in chloroform solution. Magnetic Fe dispersed in chloroform 3 o 4 The nanoparticles were transferred into the aqueous environment by binding the surfactant cetyltrimethylammonium bromide (CTAB, purchased from Sigma-Aldrich). Change the pH of the solution to alkaline by adding NaOH dropwise, then add TEOS to it, and increase the reaction temperature to 70°C to make the two self-assemble in the solution. The product was separated and washed with water, and then the CTAB template in the product was removed with acetone under an ultrasonic environment. The product afte...
Embodiment 2
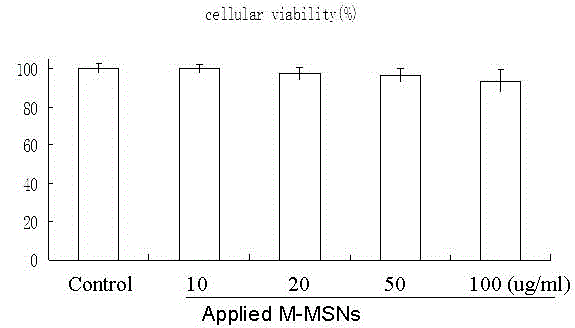
[0052] Example 2. The biocompatibility experiment of M-MSN_BACE1PEI-KALA / PHI
[0053] Cytotoxicity of M-MSN_siRNAPEI-KALA / PHI transporter: Spread Neuro2 cells on a 96-well plate with an average number of cells per well of 0.5×10 4 indivual. After incubation for 24 hours in a constant temperature incubator at 37°C, the DMEM medium (containing 10% serum and 1% double antibody, denoted as DMEM+ / +) was replaced, and different amounts of M-MSN_siRNAPEI-KALA transporter were added to the wells. Put the cell culture plate back into the incubator, and place it on the magnetic plate corresponding to the 96-well plate for 20 min. Remove the magnetic plate, continue to incubate in the cell culture incubator for 48 hours, and then add the reagents used in the CCK8 kit for counting the number of cells to each well. After further incubation at 37° C. for 2 h, the cell plate was placed in a microplate reader (Synergy2, BioTek), and the absorbance value of each well at 450 nm was read. In ...
Embodiment 3
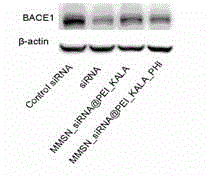
[0054] Example 3. M-MSN_BACE1PEI-KALA / PHI has a significant inhibitory effect on the expression of BACE1
[0055] The role of M-MSN_siRNAPEI-KALA / PHI transporter in interfering with the expression of BACE1:
[0056] Neuro2 cells were seeded in 6-well plates at a density of 4×10 5 / well, 37℃, 5%CO 2 Incubate under conditions for 24 hours. Using the lipofectmine2000 kit transfection method as a positive reference, the M-MSN_siRNAPEI-KALA / PHI with a concentration of 50nM siRNA was co-incubated with the cells. Put it on the ND-Fe-B magnetic plate, remove the ND-Fe-B magnetic plate after 1 hour. After 48 hours, the total cellular protein was collected. The interference effect of BACE1 was detected by western blot experiment. Such as image 3 As shown, M-MSN_siRNAPEI-KALA / PHI can significantly reduce the expression of BACE1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com