Amphipathic self-assembly ultra-short peptide nano hemostatic material
A hemostatic material and ultra-short peptide technology, applied in the field of nano-medical materials, can solve the problems of increased synthesis cost, low mechanical strength of hydrogel, etc., and achieve the effects of low synthesis cost, abundant species, and easy control of synthesis conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Amphiphilic self-assembled ultrashort peptide of the present invention, its amino acid sequence general formula is I 3 -X-Q-G-K (1-2) , as in the above formula, X is selected from Val, the number of K is 1, the N-terminal of the polypeptide is acetylated, and the C-terminal is aminated, and its amino acid sequence is as follows:
[0055] Acetylated Ac-IIIVQGK-NH2; (abbreviated as IIIVQGK)
[0056] The preparation method of the above-mentioned amphiphilic short peptide (amphiphilic short peptide sample 1):
[0057] Step 1: Distillation of dichloromethane (DMF) and dimethylformamide (DCM) solvents
[0058] Distill the purchased DMF solution under reduced pressure at 60°C, remove about 10mL of the liquid before and after distillation, and obtain pure DMF solvent; carry out vacuum distillation on the purchased DCM, first add a small amount of CaH 2 Water-absorbing agent, suspended steaming for 2-3 hours, and then distilled at atmospheric pressure to obtain pure DCM solve...
Embodiment 2
[0079] Determination of the secondary structure of the amphiphilic short peptide sample prepared by the present invention
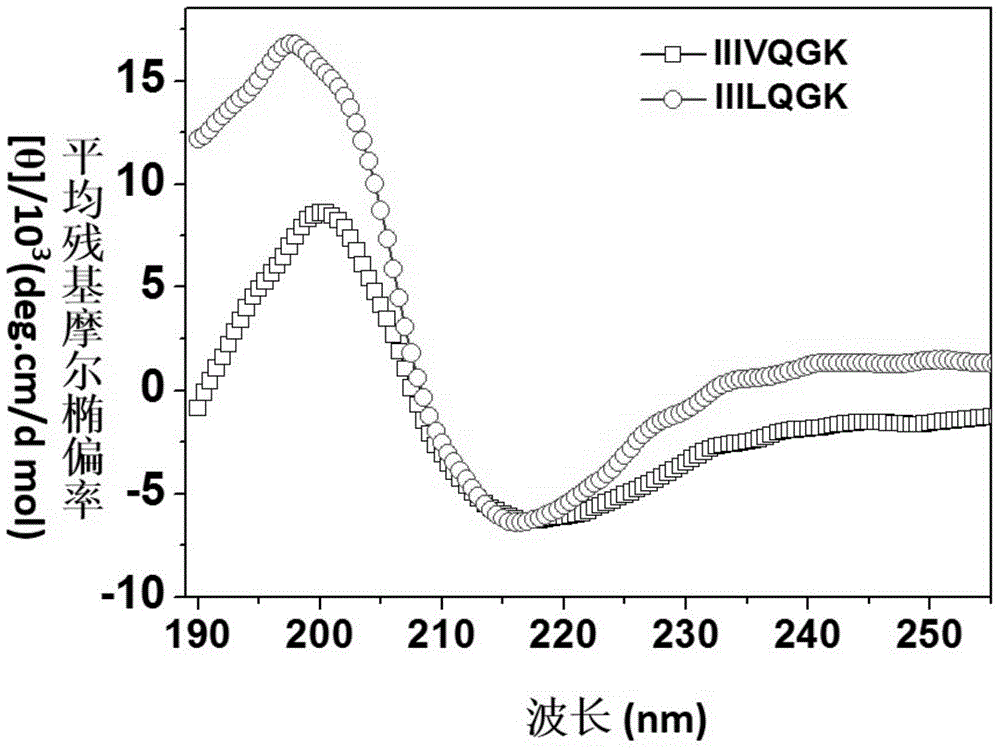
[0080] Taking the amphiphilic short peptide sample IIILQGK as an example, its secondary structure in HEPES buffer was detected by circular dichroism spectrometer. The specific measurement method is as follows:
[0081] Step 1: Preparation of HEPES buffer:
[0082] Dissolve 0.05958g of HEPES powder in 1L of ultrapure water, and adjust the pH to 7.4 with 0.4M NaOH solution.
[0083] Dissolve 6.6 mg of the amphiphilic short peptide sample IIILQGK powder in 1 mL of HEPES solution, so that the final concentration of the peptide is 8 mM, add the peptide solution to a quartz sample cell with an optical path of 0.1 mm, and scan at a wavelength of 190 nm to 250 nm. The wavelength interval is 0.1nm, the response time is 1s, all experiments are carried out at room temperature, the measurement results are as follows image 3 shown.
[0084] It can be seen from th...
Embodiment 3
[0086] Self-assembly morphology detection of amphiphilic short peptide samples IIILQGK, IIIVQGK in HEPES buffer (AFM, TEM)
[0087] The specific method is as follows:
[0088] AFM scanning: Take 10 μL of prepared peptide sample dropwise on the surface of a clean mica sheet, let it absorb for 10 seconds, and then blow dry the sample with high-purity nitrogen gas. The scanning is completed under the AFM microscope in tapping mode, and the height map and phase of the sample are obtained. In the figure, the scanning angle is 0°, and the scanning rate is 1~1.5Hz. In this experiment, an RTESP silicon probe (Veeco, Santa Barbara, CA) was used, with a tip radius of ~10 nm, a vibration arm length of 125 μm, and an elastic coefficient of 42 N / m. The same sample was scanned 5 times at different positions, and the results showed that IIILQGK and IIIVQGK self-assembled into fiber structures in HEPES solution ( figure 2 a and Figure 4 a)
[0089] TEM: Take a drop of polypeptide soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mechanical strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com