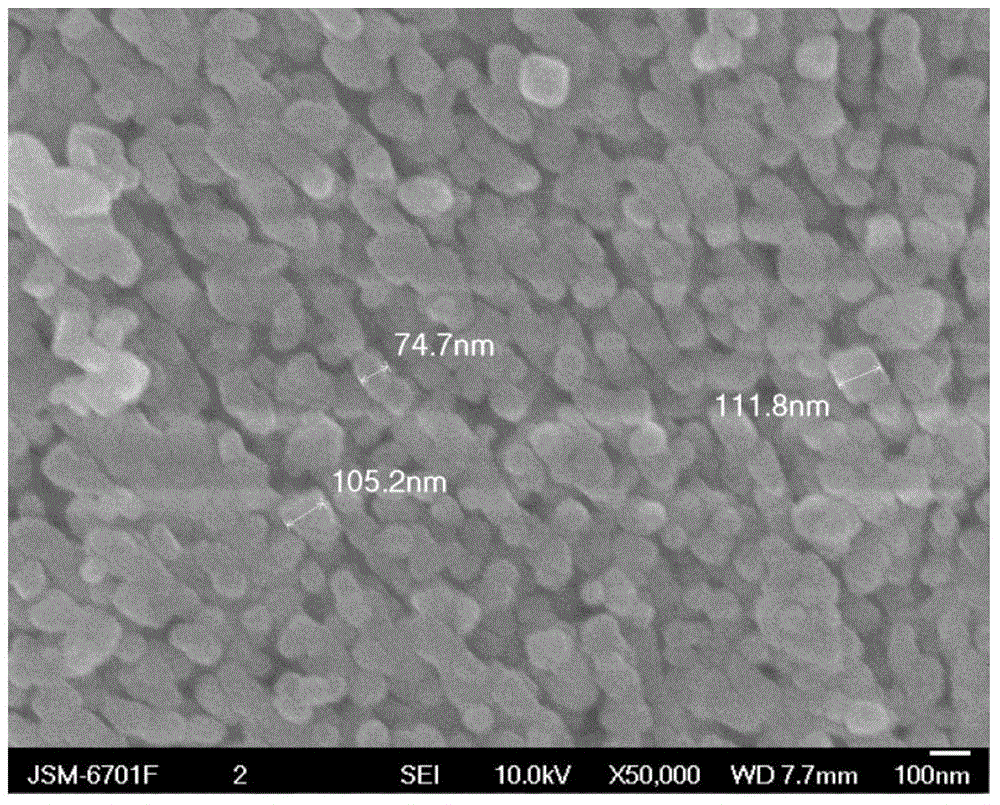
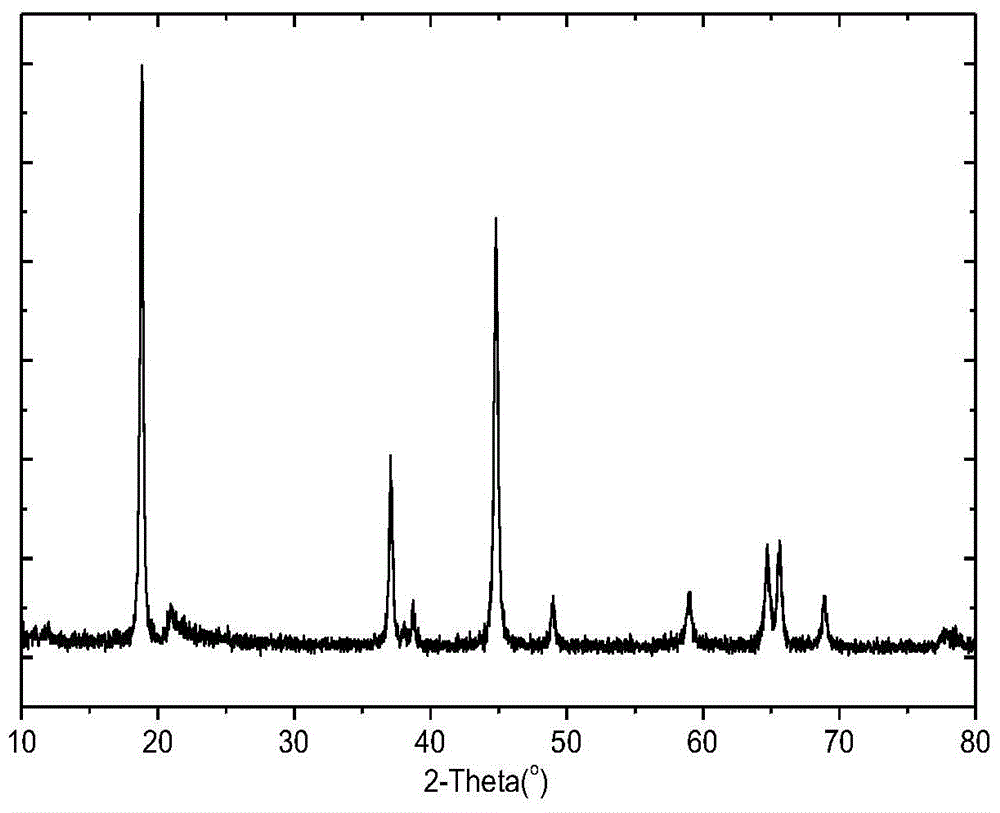
Preparation method of high-capacity lithium-rich anode material
A cathode material and lithium salt technology, which is applied in the field of high-capacity lithium-rich cathode materials and their preparation, can solve the problems of insufficient compactness, large specific surface area of materials, and uneven sintering of materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1. Preparation of high-capacity lithium-rich cathode material
[0023] The precursor was nickel sulfate hexahydrate (NiSO 4 .6H 2 O), manganese sulfate pentahydrate (MnSO 4 .5H 2 O), cobalt sulfate heptahydrate (CoSO 4 .7H 2 O) be made into 2M transition metal solution, with 2M sodium carbonate (Na 2 CO 3 ) as a precipitant, 0.2M ammonia water as a buffer, and obtained after co-precipitation at 60° C., pH=7-8, washing, filtering and drying. The precursor obtained above was pre-sintered in a muffle furnace, raised to 500°C at a rate of 5°C / min in an air atmosphere, kept at a constant temperature for 5h, and then naturally cooled to room temperature to obtain an intermediate. The number of moles of transition metals in the above intermediates is denoted as M 1 , then the intermediate is mixed with lithium carbonate and molten salt sodium chloride in molar ratio M 1 :Li:Na=1:1.5:2 mixed evenly, and sintered again under air atmosphere. The sintering steps ...
Embodiment 2
[0031] Other conditions are identical with embodiment 1, and difference is the mol ratio of intermediate obtained in step 1) and sodium chloride, i.e. M 1 : Li:Na=1:1.5:4, wherein M is a transition metal element. The positive and negative terminals of the button battery. Electrolyte solution and battery assembly are identical with embodiment 1, and gained lithium-rich positive electrode material is at 0.05C (corresponding to 12.5mAg -1 ) is shown in Table 1 for the first cycle charge and discharge capacity. The material is at 0.05C (equivalent to 12.5mAg -1 ) rate, the voltage range is 2-4.7V, the test temperature is 25°C, and the specific discharge capacity is 270mAhg -1 , Coulombic efficiency is 87%. Compared with Example 1, the specific capacity is slightly worse.
Embodiment 3
[0033] Other conditions are identical with embodiment 1, and difference is the mol ratio of intermediate obtained in step 1) and sodium chloride, i.e. M 1 : Li:Na=1:1.5:6, wherein M is a transition metal element. The positive pole, negative pole, electrolyte and battery assembly of button cell are identical with embodiment 1, and gained lithium-rich positive electrode material is at 0.05C (corresponding to 12.5mAg -1 ) rate of the first cycle charge and discharge capacity shown in Table 1. The material is at 0.05C (equivalent to 12.5mAg -1 ) rate, the voltage range is 2-4.7V, the test temperature is 25°C, and the specific discharge capacity is 266mAhg -1, Coulombic efficiency is 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com