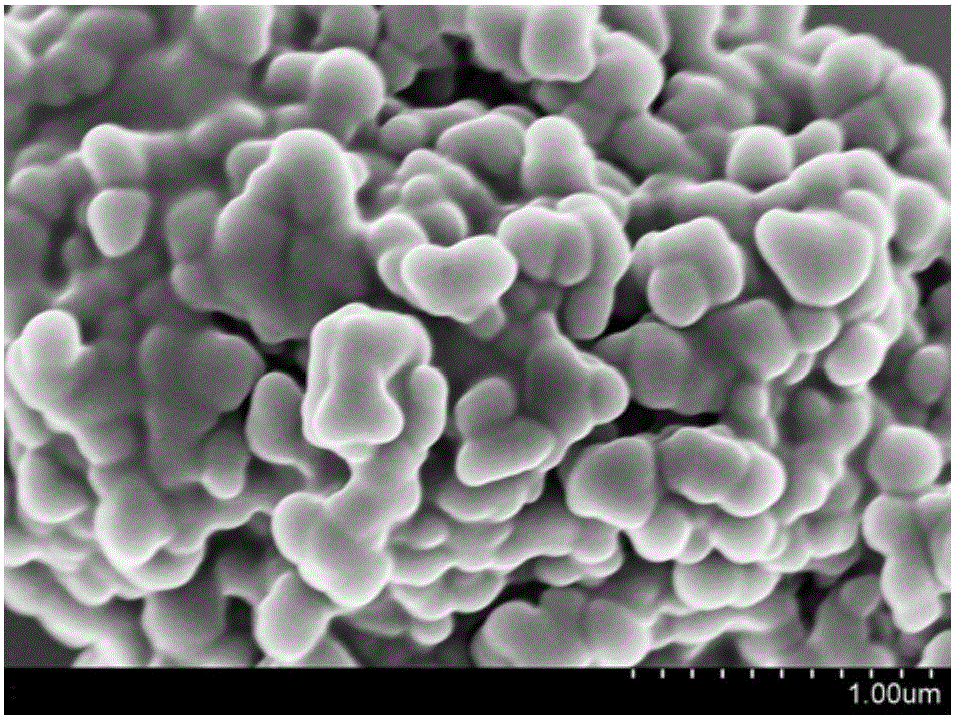
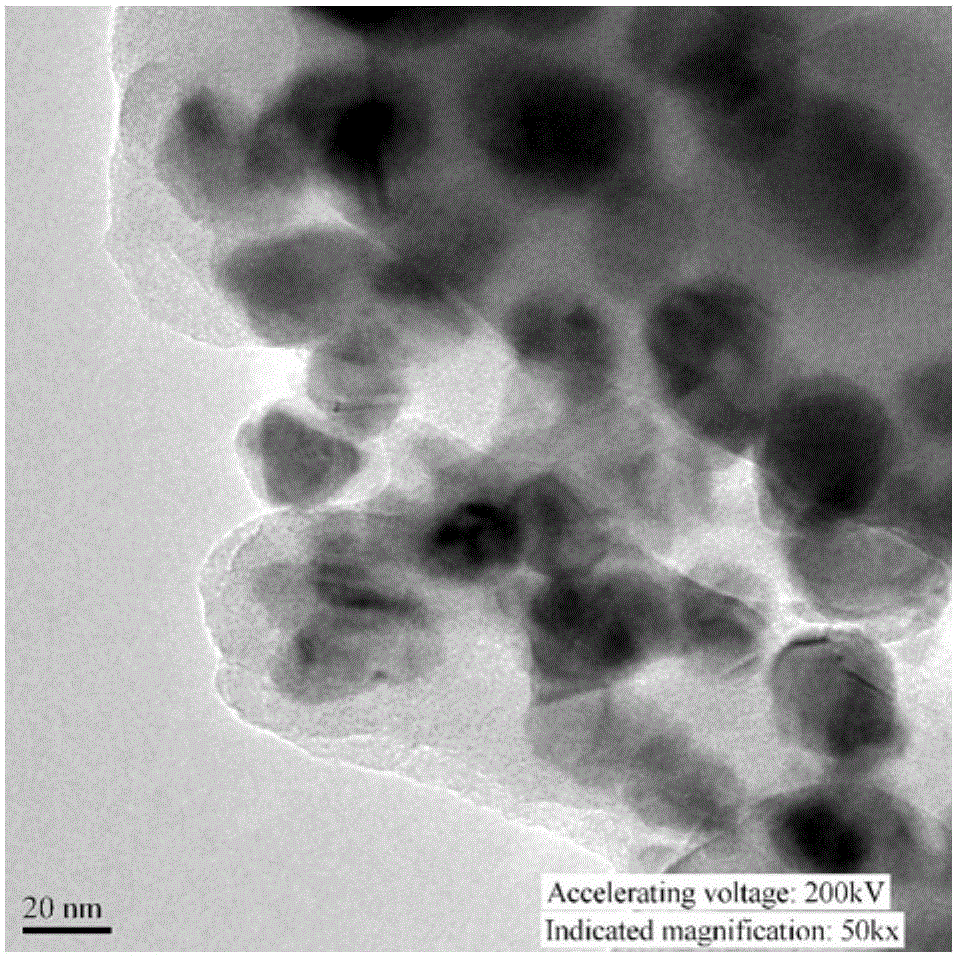
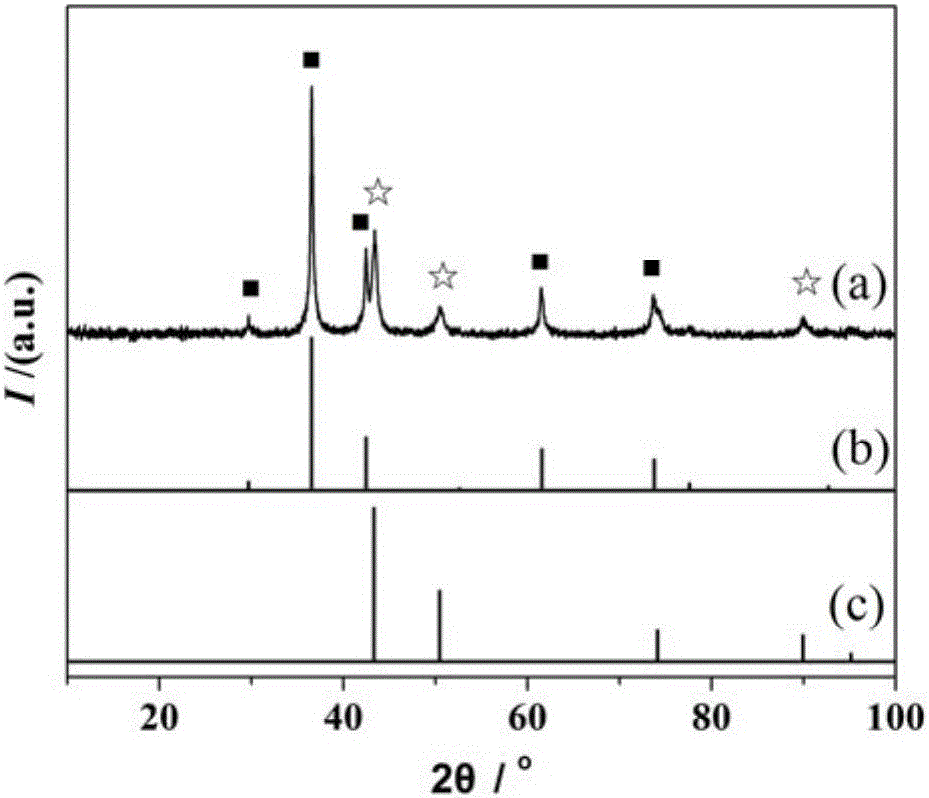
Preparation method for copper/cuprous oxide/cyclized polyacrylonitrile visible-light-driven photocatalyst
A technology of polyacrylonitrile and cuprous oxide, which is applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, and can solve the problems of difficult mixing, high production cost, and consumption Time and other issues, to achieve the effect of reducing photocorrosion, improving stability, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 0.20 g of polyacrylonitrile solid and dissolve it in 10.0 mL of dimethyl sulfoxide, and stir under airtight until completely dissolved. In addition, 3.36g of copper nitrate trihydrate (Cu(NO 3 ) 2 ·3H 2O) Dissolve in 20.0 mL of dimethyl sulfoxide. Mix the obtained copper nitrate solution and polyacrylonitrile solution evenly under stirring to obtain a dimethyl sulfoxide mixed solution containing both copper nitrate and polyacrylonitrile. Weigh 1.41 g of solid sodium hydroxide and dissolve it in 100 mL of distilled water to form a sodium hydroxide solution, and add the above-mentioned mixed solution of dimethyl sulfoxide to the sodium hydroxide solution dropwise under stirring for 30 minutes. After the dropwise addition, the stirring was continued for 30 minutes to obtain a nano-copper hydroxide / polyacrylonitrile suspension. Then dissolve 1.81 g of ascorbic acid in 50 mL of distilled water to obtain an ascorbic acid solution, and add dropwise to the above-menti...
Embodiment 2
[0043] Weigh 0.20 g of polyacrylonitrile solid and dissolve it in 5.0 mL of dimethyl sulfoxide, and stir until completely dissolved. Another 4.69 g of copper nitrate trihydrate was weighed and dissolved in 20.0 mL of dimethyl sulfoxide. Mix the obtained copper nitrate solution and polyacrylonitrile solution evenly under stirring to obtain a dimethyl sulfoxide mixed solution containing both copper nitrate and polyacrylonitrile. Weigh 1.80 g of solid sodium hydroxide and dissolve it in 100 mL of distilled water to form a sodium hydroxide solution, and add the above-mentioned mixed solution of dimethyl sulfoxide to the sodium hydroxide solution dropwise under stirring for 40 minutes. After the dropwise addition, the stirring was continued for 60 minutes to obtain a nano-copper hydroxide / polyacrylonitrile suspension. Then 2.00 g of ascorbic acid was dissolved in 50 mL of distilled water to obtain an ascorbic acid solution, which was added dropwise to the above-mentioned nano-copp...
Embodiment 3
[0045] Weigh 0.20 g of polyacrylonitrile solid and dissolve it in 10.0 mL of dimethyl sulfoxide, and stir under airtight until completely dissolved. Another 3.36 g of copper nitrate trihydrate was weighed and dissolved in 20.0 mL of dimethyl sulfoxide. Mix the obtained copper nitrate solution and polyacrylonitrile solution evenly under stirring to obtain a dimethyl sulfoxide mixed solution containing both copper nitrate and polyacrylonitrile. Weigh 1.41 g of solid sodium hydroxide and dissolve it in 100 mL of distilled water to form a sodium hydroxide solution, and add the above-mentioned dimethyl sulfoxide mixed solution dropwise to the sodium hydroxide solution under stirring for 60 minutes. After the dropwise addition, the stirring was continued for 60 minutes to obtain a nano-copper hydroxide / polyacrylonitrile suspension. Then dissolve 1.81 g of ascorbic acid in 50 mL of distilled water to obtain an ascorbic acid solution, and add dropwise to the above-mentioned nano-copp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com