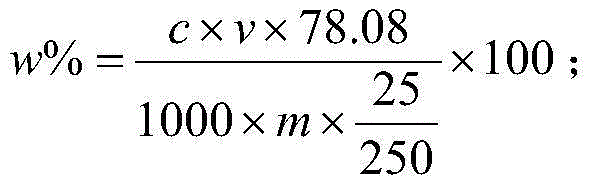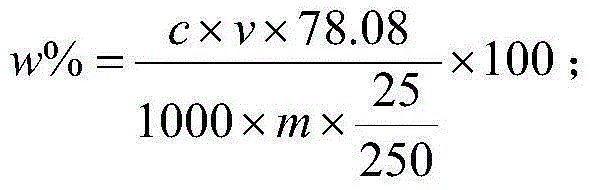Method for determining content of calcium fluoride in fluorite
A technology for medium calcium fluoride and fluorine content, which can be used in chemical analysis by titration, material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, etc., which can solve the problem of low precision. , Introduce problems such as large errors and low calcium fluoride results to achieve the effect of improving accuracy and precision, good reduction and masking, and clear discoloration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] For a fluorite standard sample BH0121-17W (CaF 2 standard value 98.59%) to measure:
[0022] (1) Weigh 0.2000g sample and place it in a 250mL beaker, add 5mL of calcium-containing acetic acid solution, the concentration of calcium-containing acetic acid solution is 2.0mg calcium per milliliter, cover with a watch glass, shake the beaker to distribute the sample evenly, heat Slightly boil for 2 minutes, keep warm for 2 minutes, then immediately filter into a 250mL beaker with slow filter paper, wash the beaker and the precipitate with hot (60-70°C) fluorine-containing washing solution with a concentration of 10μg / mL for 4 times;
[0023] (2) Put the slow-speed filter paper and the precipitate in a platinum crucible, ash and burn in a muffle furnace, take it out, cool it, add 3.2g of sodium carbonate and boric acid in a mass ratio of 2:1 mixed flux, mix well , placed in a high-temperature furnace at a temperature of 500°C, heated to 950°C and melted for 20 minutes, taken...
Embodiment 2
[0026] For a fluorite standard sample YSB14785-02 (CaF 2 standard value 93.28%) to measure:
[0027] (1) Weigh 0.5000g sample and place it in a 250mL beaker, add 10mL of calcium-containing acetic acid solution, the concentration of calcium-containing acetic acid solution is 0.8mg calcium per milliliter, cover with a watch glass, shake the beaker to make the sample evenly distributed, room temperature Stand for 30 minutes, stir once every 5 minutes, then immediately filter into a 250mL beaker with slow filter paper, wash the beaker and the precipitate with hot (60-70°C) fluorine-containing washing solution with a concentration of 20μg / mL for 5 times;
[0028] (2) Put the slow filter paper together with the precipitate in a platinum crucible, incinerate and burn in a muffle furnace, take it out, cool it down, add 5g of sodium carbonate and boric acid in a mass ratio of 2:1 to obtain a mixed flux, mix well, and place In a high-temperature furnace with a temperature of 500°C, hea...
Embodiment 3
[0031] For CaF in a fluorite sample 2 The content is measured:
[0032] (1) Weigh 0.4000g sample and place it in a 250mL beaker, add 10mL calcium-containing acetic acid solution, the concentration of calcium-containing acetic acid solution is 1.2mg calcium per milliliter, cover with a watch glass, shake the beaker to distribute the sample evenly, heat Slightly boil for 5 minutes, keep warm for 2 minutes, then immediately filter into a 250mL beaker with slow filter paper, wash the beaker and the precipitate with hot (60-70°C) fluorine-containing washing solution with a concentration of 15μg / mL for 6 times;
[0033] (2) Place the slow filter paper together with the precipitate in a platinum crucible, incinerate and burn in a muffle furnace, take it out, cool it down, add 4.5g of sodium carbonate and boric acid in a mass ratio of 2:1 to obtain a mixed flux, mix well, Place in a high-temperature furnace at 500°C, heat up to 950°C and melt for 15 minutes, take it out, cool, then p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com