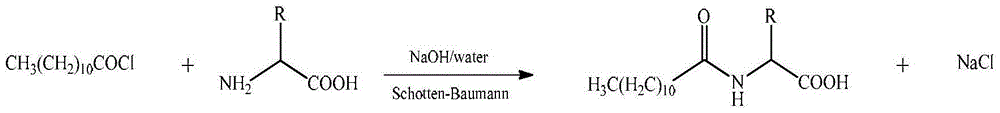
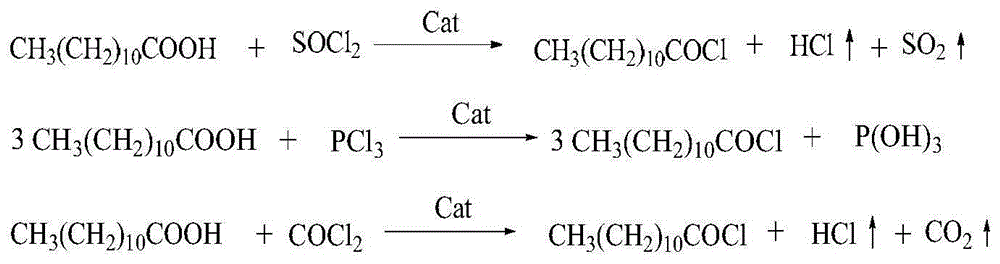
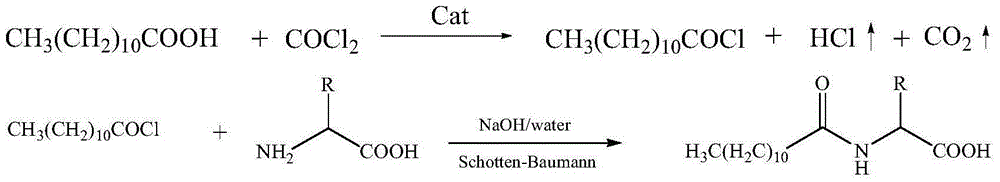
Method for synthesizing laurel acyl amino acid sodium
A technology of sodium lauroyl amino acid and synthesis method, applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of incomplete reaction of long-chain fatty acids, affecting product quality, and high long-chain fatty acids, Achieve the effect of solving difficult recovery, improving conversion rate and low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In a 500mL four-necked flask equipped with a thermometer, a reflux condenser, a gas pipe, and stirring, add 200g (1mol) of lauric acid, 1.5g (0.02mol) of N-methyl-N-phenylformamide, and heat up to 60°C , control the reaction temperature to be 70~80 ℃, in 5~10 hours, pass into 110g (1.1mol) of phosgene, the reaction is completed, the reaction is incubated for 2 hours, the nitrogen gas is driven to the tail gas pH value is neutral, and 218.5g of lauroyl chloride is obtained , the yield is 99%.
[0040] parameter
Indicator
Exterior
light yellow liquid
[0041] Lauroyl chloride (%)
99.5
Lauric acid (%)
0.1
Lauric anhydride (%)
0.1
[0042] In a 1000mL four-necked flask equipped with a thermometer, dropping funnel and stirring, add 186g (1.1mol) of sodium glutamate, dissolve in 570g of water, stir at room temperature to dissolve all of it, and prepare a 25% aqueous solution. 219g (1mol) of lauroyl ch...
Embodiment 2
[0045] The synthesis of lauroyl chloride is the same as that in Example 1.
[0046] In a 1000mL four-necked flask equipped with a thermometer, dropping funnel and stirring, add 122g (1.1mol) of sodium sarcosinate, dissolve in 200g of water, stir at room temperature to dissolve all, then add 166g of acetone to make 25 % acetone-water solution, put 219g (1mol) of lauroyl chloride into a dry dropping funnel, control the temperature in an ice-water bath to be 15-20°C, add lauroyl chloride under stirring, and dropwise add 25% aqueous sodium hydroxide solution , so that the reaction solution is kept at pH=8~10 until the addition is completed; the reaction is continued for 4~5h. After the reaction, the aqueous acetone phase was extracted with 500 mL of ethyl acetate, the layers were separated, and the organic phase was collected. The pH value was adjusted to 1-2 with 6N hydrochloric acid, and a large number of white crystals were precipitated. After all the product was precipitated,...
Embodiment 3
[0049] The synthesis of lauroyl chloride is the same as that in Example 1.
[0050] In a 1000mL four-necked flask equipped with a thermometer, dropping funnel and stirring, add 107g (1.1mol) of sodium glycinate, dissolve it in 321g of water, stir at room temperature to make it completely dissolved, then prepare a 25% aqueous solution, add 219g of lauroyl chloride (1mol) is put into a dry dropping funnel, the temperature is controlled to be 15~20° C. in an ice-water bath, lauroyl chloride is added under stirring, and a 25% aqueous sodium hydroxide solution is added dropwise simultaneously, so that the reaction solution is always at pH=8 ~10, until the addition is complete; continue the reaction for 4~5h. After the reaction, extract with 500 mL of ethyl acetate, separate the layers, and collect the organic phase. The pH value of the aqueous phase was adjusted to 1-2 with 6N hydrochloric acid, and a large number of white crystals were precipitated. After all the product was prec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com