Anionic polyelectrolyte and nylon fabric after-finish flame retarding method
A technology of polyelectrolyte and nylon fabric, which can be used in fiber processing, textiles and papermaking, and can solve the problems of poor durability and low flame retardant efficiency of polymer electrolytes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] The present invention also provides a method for preparing the above-mentioned anionic polyelectrolyte, comprising: combining hypophosphorous acid raw materials with R 1 =O, H-R 2 -H, POM and Cl-R 3 -One or more reactions in Cl to obtain anion polyelectrolyte; the hypophosphorous acid raw material is hypophosphorous acid and / or hypophosphite salt.
[0076] where the R 1 , R 2 with R 3 All are the same as above, and will not be repeated here.
[0077] According to the invention, it is preferred to combine hypophosphorous acid and / or hypophosphite with R 1 =O, H-R 2 -H reaction to obtain anionic polyelectrolyte, or hypophosphorous acid and / or hypophosphite with polyoxymethylene, Cl-R 3 -Cl reaction to obtain anionic polyelectrolyte.
[0078] When hypophosphorous acid and / or hypophosphite with R 1 =O, H-R 2 When -H reacts, its reaction chemical formula is as follows:
[0079]
[0080] When hypophosphorous acid and / or hypophosphite and polyoxymethylene, Cl-R ...
Embodiment 1
[0096] 1.1 Add 70.0g of sodium hypophosphite, 36.0g of paraformaldehyde, 300ml of deionized water and 20ml of hydrochloric acid into the reaction kettle, turn on the mechanical stirring and raise the reaction temperature to 90°C, keep it warm and react for 24h, then obtain a white color by rotary evaporation The solid product is sodium hydroxymethyl hypophosphite.
[0097] 1.2 Add 13g of sodium hydroxymethyl hypophosphite obtained in 1.1, 13g of itaconic acid, 20g of phenylphosphonic dichloride, 20ml of deionized water and 150ml of acetonitrile into the reactor, turn on mechanical stirring and raise the reaction temperature to 80 °C, condense and reflux to discharge the hydrogen chloride produced in the reaction, keep warm and react for 6h, then obtain the product by rotary evaporation, wash with ether to remove impurities, and dry to obtain a white solid product, which is an anionic polyelectrolyte. The chemical reaction formula is as follows:
[0098]
[0099] Among them,...
Embodiment 2
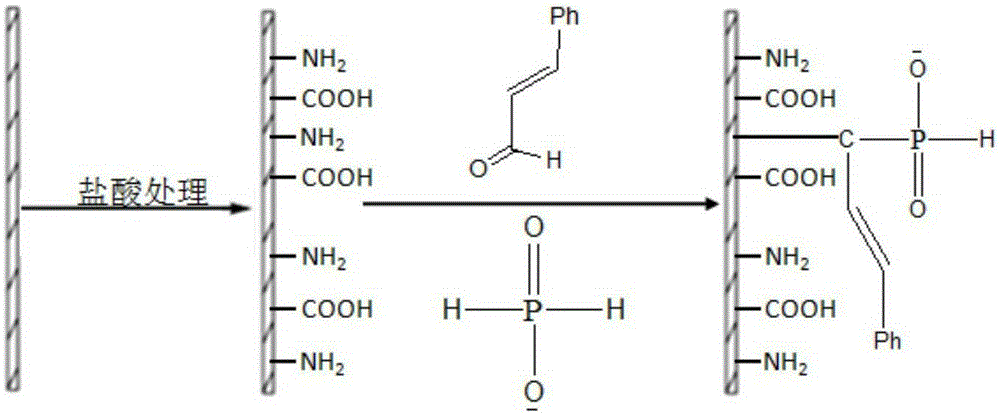
[0102] Add 20.0g of chitosan, 12.0g of phenylphosphonic dichloride, 4.4g of acrylic acid, 10.0g of triethylamine and 300ml of acetonitrile into the reaction kettle, turn on the mechanical stirring and raise the temperature to 60°C, keep warm and react for 12h, then rotate evaporate and wash , filtered, and dried to obtain a yellow solid product, which is modified chitosan.
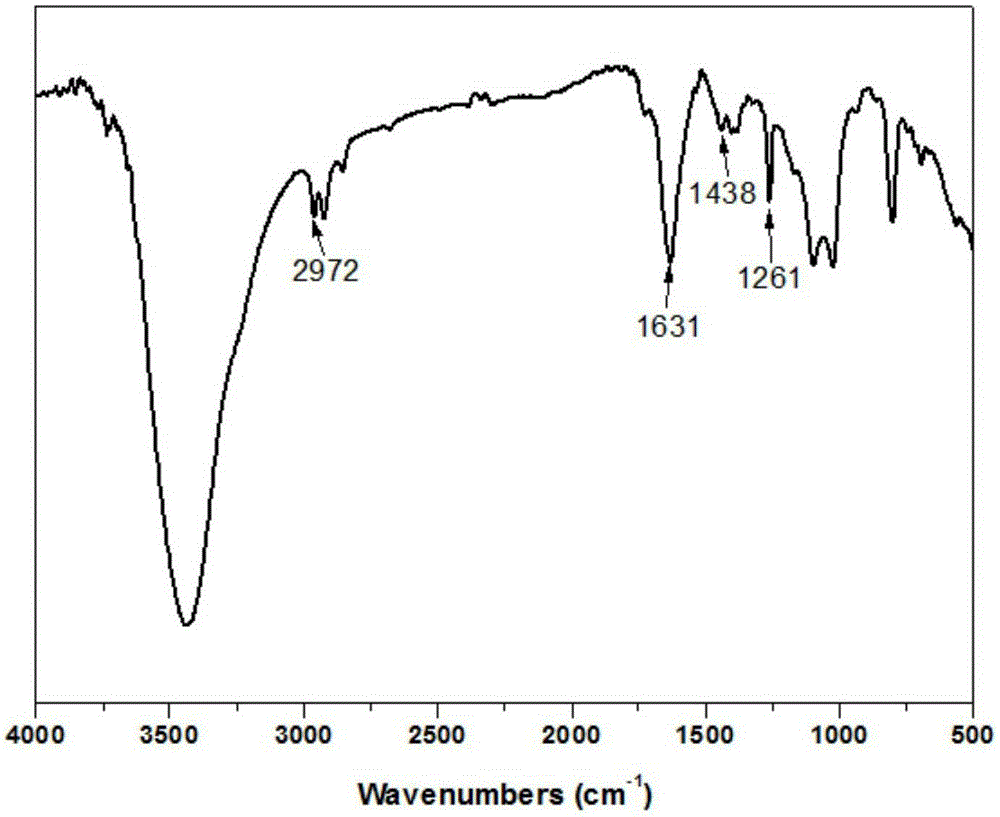
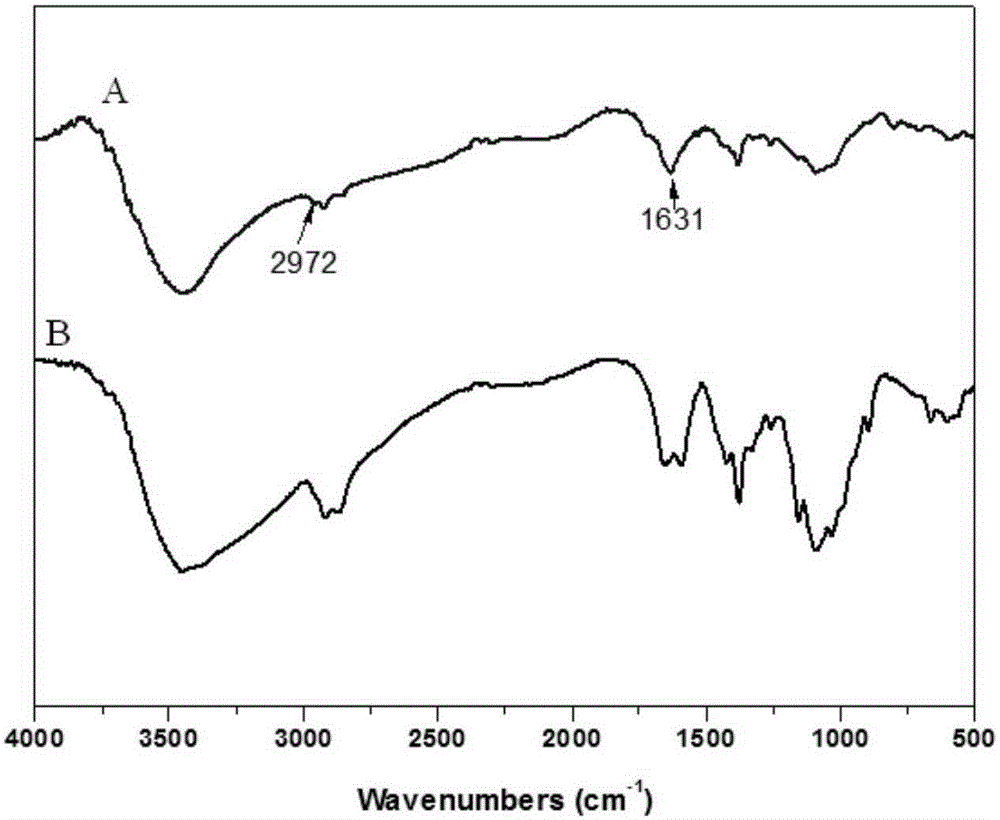
[0103] Utilize infrared spectrum to analyze chitosan and modified chitosan in embodiment 2, obtain its infrared spectrum figure such as figure 2 Shown, wherein A is modified chitosan, B is chitosan. By comparing the spectra of chitosan and modified chitosan, it can be found that 1631cm -1 and 2972cm -1 The peaks at correspond to the characteristic peaks of the double bond and the methylene connected to it, indicating that acrylic acid has been grafted onto the chitosan structure through phenylphosphonyl dichloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com