1,2,3-Triazole unit-based micro-molecular luminescent material and application thereof
A technology of luminescent materials and small molecules, applied in luminescent materials, electrical components, circuits, etc., can solve the problems of low exciton utilization of ordinary fluorescent materials, and achieve molecular weight determination, low sublimation temperature, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] This example prepares the small molecule luminescent material P1, and its reaction is shown in the following formula:
[0027]
[0028] The specific implementation steps are: under a nitrogen atmosphere, add intermediate 1 (4,5-bis(4-bromophenyl)-2-phenyl-2H-1,2,3-triazole) to a 20mL three-necked flask ( 1.365g, 3mmol) and diphenylamine (1.268g, 7.5mmol), then add 15mL of 1,3-dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone, stir, after being completely dissolved, go to Add ethylene iodide (1.433g, 7.5mmol) potassium carbonate (2.070g, 15mmol), 1,3-dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone 1,4,7 , 10,13,16-hexaoxacyclooctadecane (0.792g, 3mmol), heated to 190°C, reacted for 24 hours, cooled the reaction system to room temperature, extracted with dichloromethane, and used petroleum ether: dichloromethane = 2:1 ratio of eluent was subjected to silica gel column chromatography to obtain 806 mg of white solid with a yield of 42.67%. C 44 h 33 N 5 M / S=631.27 Theoretical v...
Embodiment 2
[0033] This example prepares the small molecule luminescent material P2, and its reaction is shown in the following formula:
[0034]
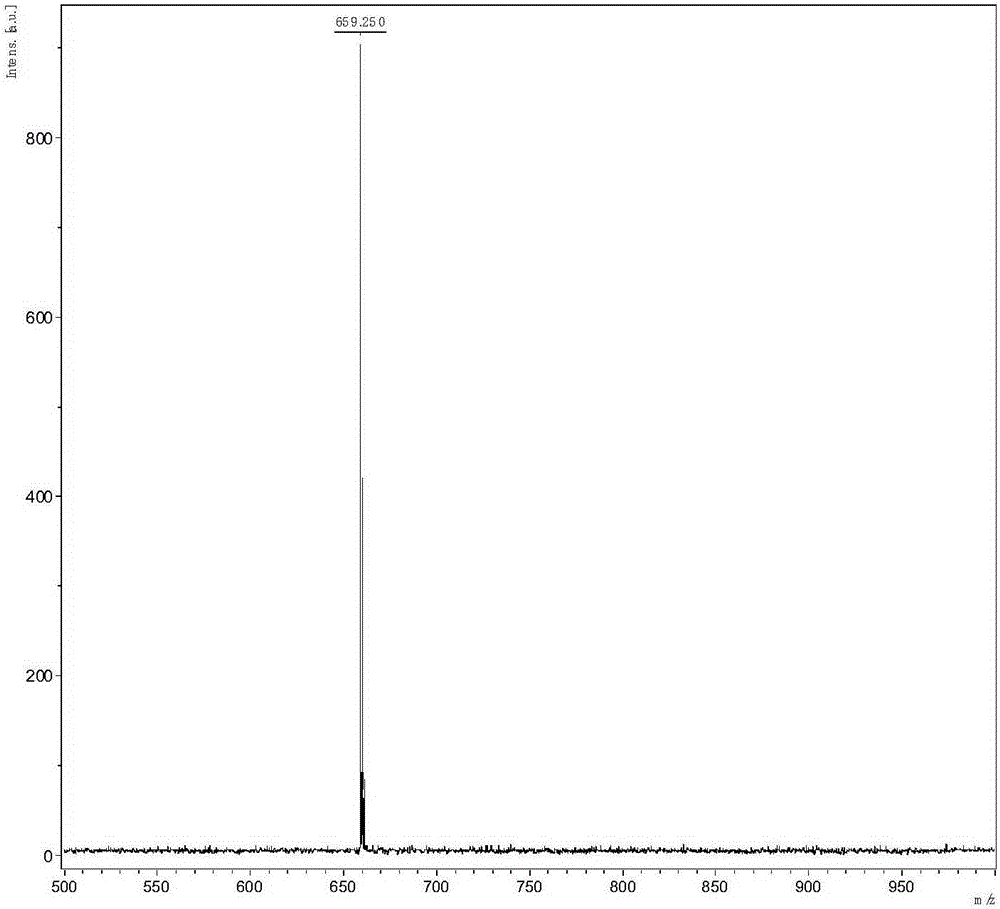
[0035] The specific implementation steps are as follows: the diphenylamine in Example 1 is replaced with equivalent 4,4'-di-tert-butyldiphenylamine, and other raw materials and steps are the same as in Example 1 to obtain 1345 mg of white solid product with a yield of 48.87%. . C 60 h 65 N 5 M / S=855.52m / z: 855.52 (100.0%), 856.53 (65.6%), 857.53 (21.2%), 858.53 (4.7%), 856.52 (1.8%), 857.52 (1.2%) C, 84.17; H, 7.65; N, 8.18.
Embodiment 3
[0037] This example prepares the small molecule luminescent material P3, and its reaction is shown in the following formula:
[0038]
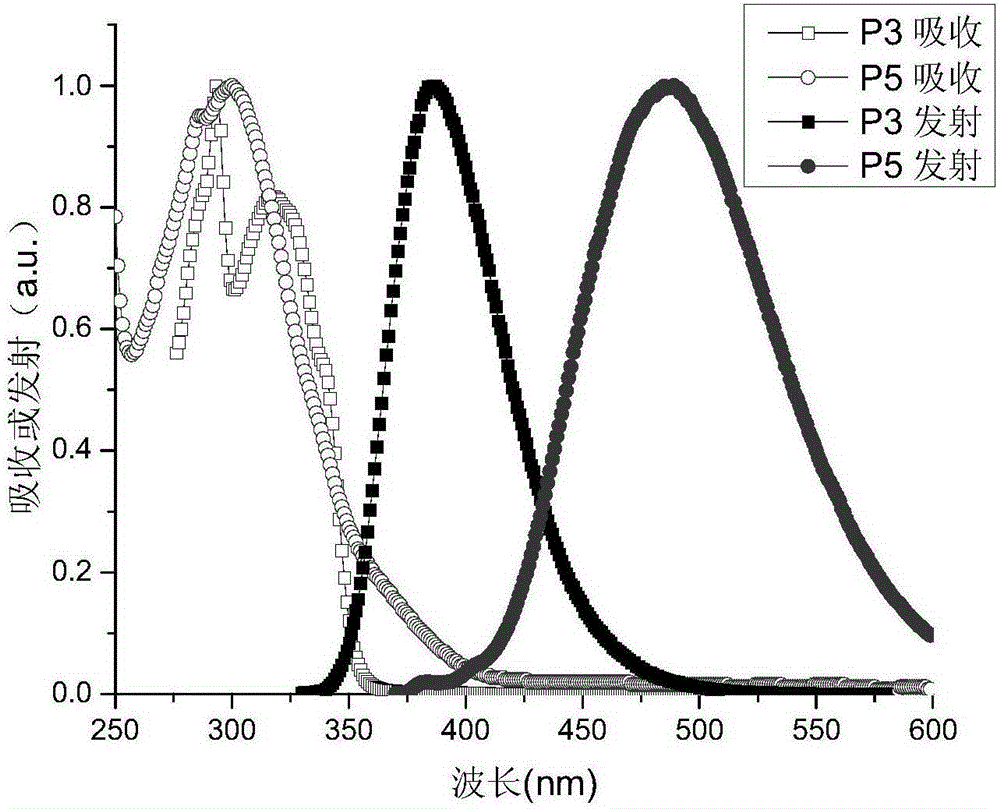
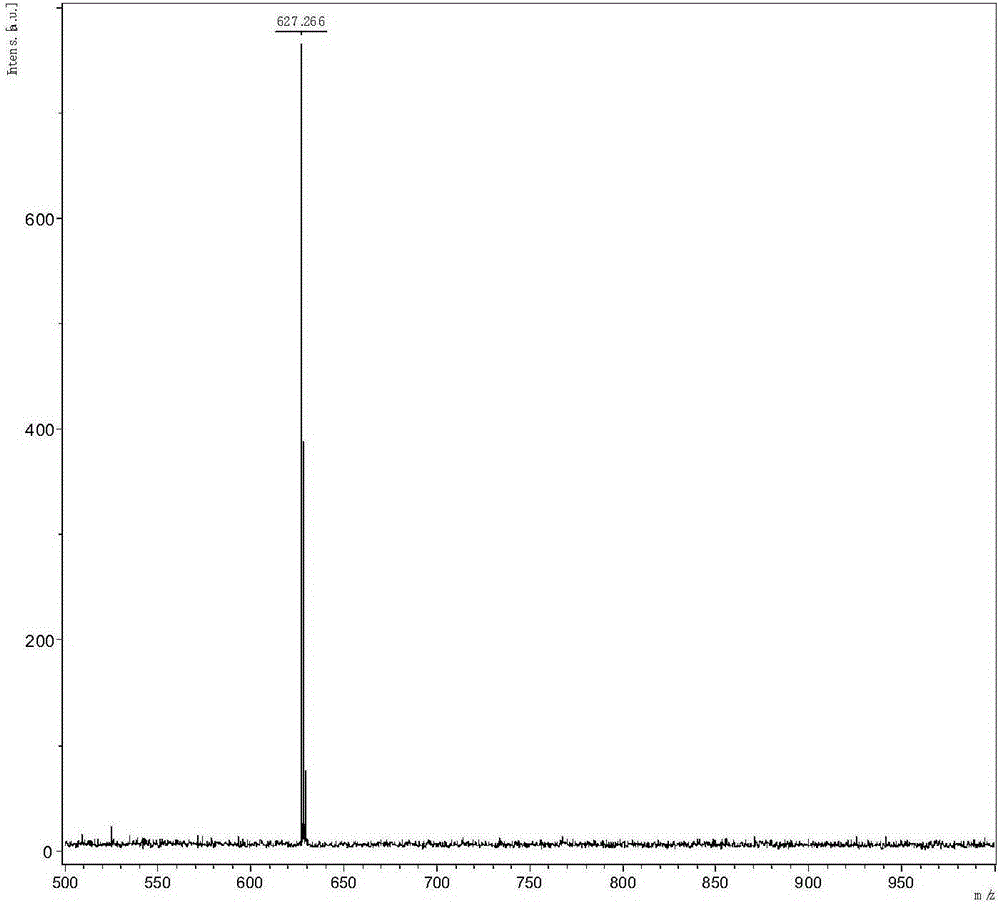
[0039] The specific implementation steps are as follows: the diphenylamine in Example 1 is replaced by an equivalent amount of carbazole, and other raw materials and steps are the same as in Example 1 to obtain 872 mg of a white solid product with a yield of 46.29%. C 44 h 29 N 5 M / S=627.24 Theoretical values: 627.24 (100.0%), 628.25 (47.9%), 629.25 (11.2%), 630.25 (1.9%), 628.24 (1.8%). C, 84.19; H, 4.66; N, 11.16.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com