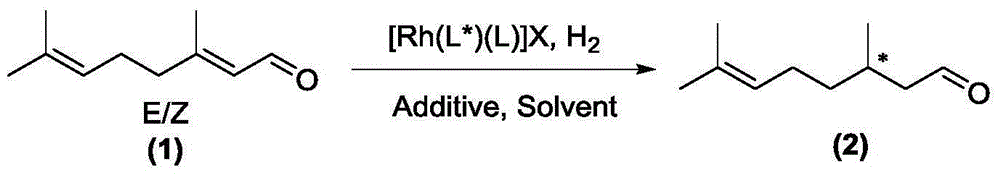Method for preparing chiral citronellal through citral catalytic asymmetric hydrogenation
A catalytic hydrogenation, asymmetric technology, applied in the field of flavors and fragrances, can solve the problems of high catalyst consumption, difficult industrial application, cumbersome process, etc., and achieve the effects of high yield, easy separation, and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0041] The embodiments of the present invention are described in detail below. This embodiment is implemented on the premise of the technical solution of the present invention, and detailed implementation methods and specific operating procedures are provided, but the protection scope of the present invention is not limited to the following implementation example.
[0042] In the following examples, the chiral rhodium complex used after coordination preparation in the following examples is expressed as [Rh(L*)(L)]X, and can also be obtained by rhodium salts with anion and auxiliary ligand [ Rh(L) 2 ]X or [RhX(L)] 2 In situ coordination synthesis with chiral ligand L*.
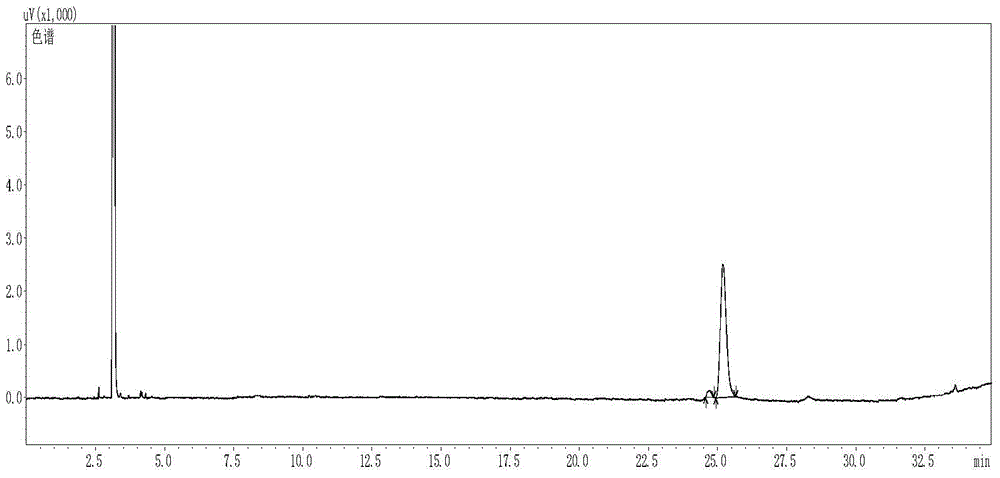
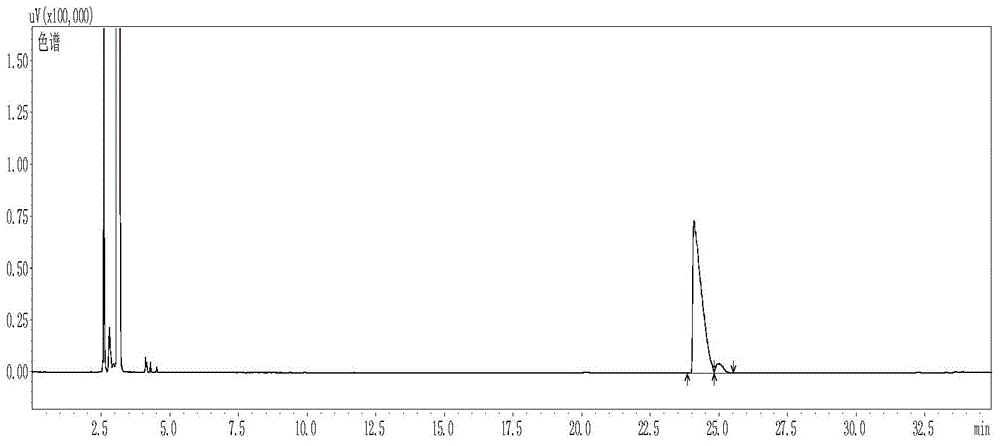
[0043] Gas chromatograph: Agilent7890, column Supelcoβ-DEX TM 225, inlet temperature: 220°C; split ratio 50:1; heating program: initial temperature 100°C; rise to 120°C at a rate of 5°C / min, hold for 0min; rise to 200°C at a rate of 20°C / min , keep for 7min, detector temperature: 300°C;
[0044] as attache...
Embodiment 1
[0047] Preparation of chiral citronellal
[0048] In a 10 mL reaction tube, add the phosphine ligand R-L1a (3.5 mg, 0.005 mmol) and bis(1,5-cyclooctadiene) rhodium tetrafluoroborate [Rh(COD) 2 ] BF 4 (2.1mg, 0.005mmol), the system passed through the vacuum line, replaced with nitrogen three times, added freshly steamed degassed toluene (2mL), the solution was stirred at room temperature for 1 hour, and the solvent was removed under reduced pressure to obtain a brown solid, which was vacuum pumped for 2 After 1 hour, 2 mL of methanol solvent was added, and this solution was added to BF containing E-citral (76.1 mg, 0.50 mmol, E / Z=99 / 1, chiral rhodium complex [Rh(R-L1a)(COD)] 4 The molar ratio of citral is 1 / 100) and sodium iodide (7.5mg, 0.05mmol) into a vial, put it into an autoclave, and after 6 hydrogen replacements, the initial hydrogen pressure is 20bar, and the reaction is stirred at 15°C for 24 Hour. Cool, release gas carefully, open the autoclave, take out the vial, ...
Embodiment 2
[0050] Preparation of chiral citronellal
[0051] In a 10mL reaction tube, add the phosphine ligand S-L1b (4.1mg, 0.005mmol) and rhodium bis(1,5-cyclooctadiene)tetrafluoroborate [Rh(COD) 2 ] BF 4 (2.1mg, 0.005mmol), the system passed through the vacuum line, replaced with nitrogen three times, added freshly steamed degassed toluene (2mL), the solution was stirred at room temperature for 1 hour, and the solvent was removed under reduced pressure to obtain a brown solid, which was vacuum pumped for 2 After 1 hour, add 2mL of toluene solvent, and add this solution into BF containing E-citral (761mg, 5mmol, E / Z=99 / 1, chiral rhodium complex [Rh(R-L1b)(COD)] 4 The molar ratio with citral is 1 / 1000) and potassium iodide (42mg, 0.25mmol) in a vial, put into an autoclave, and after 6 times of hydrogen replacement, make the initial hydrogen pressure 35bar, and stir and react at 35°C for 10 hours. Cool, release gas carefully, open the autoclave, take out the vial, drain the solvent, NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com