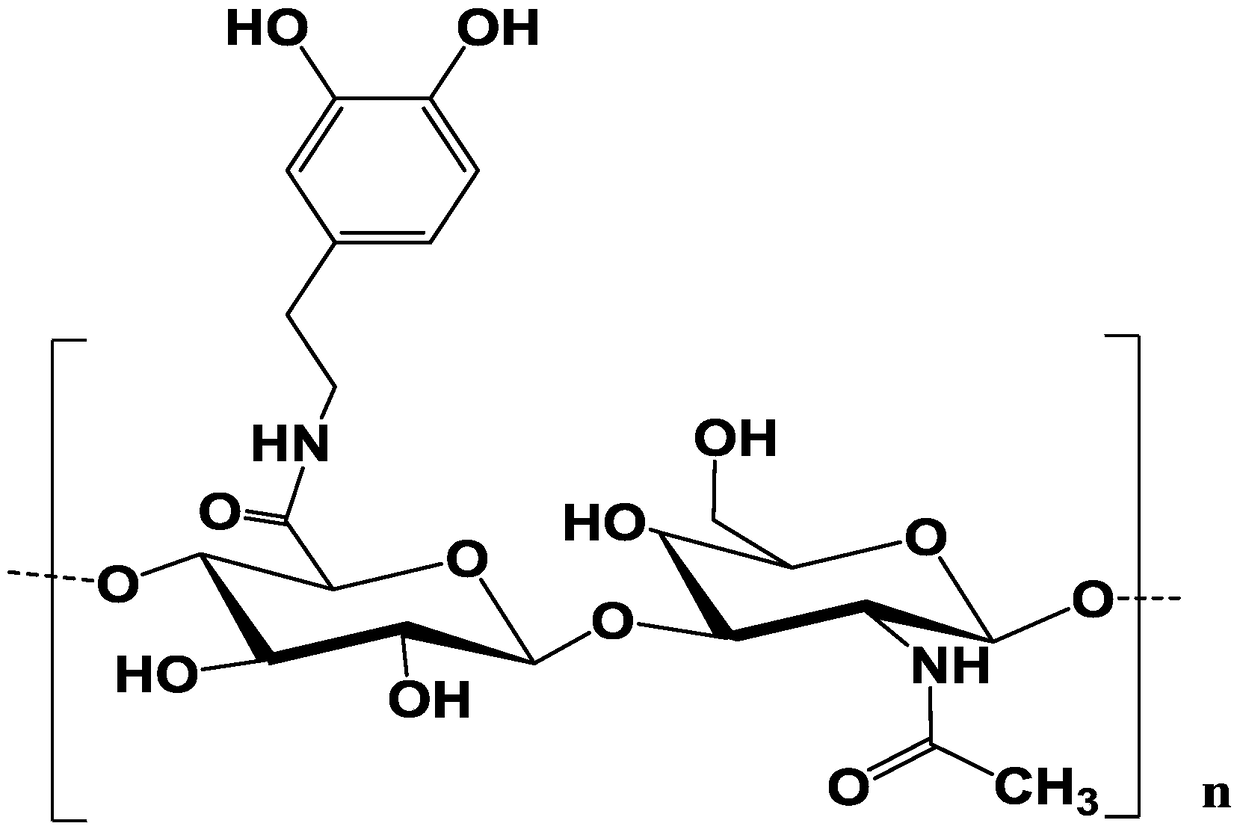
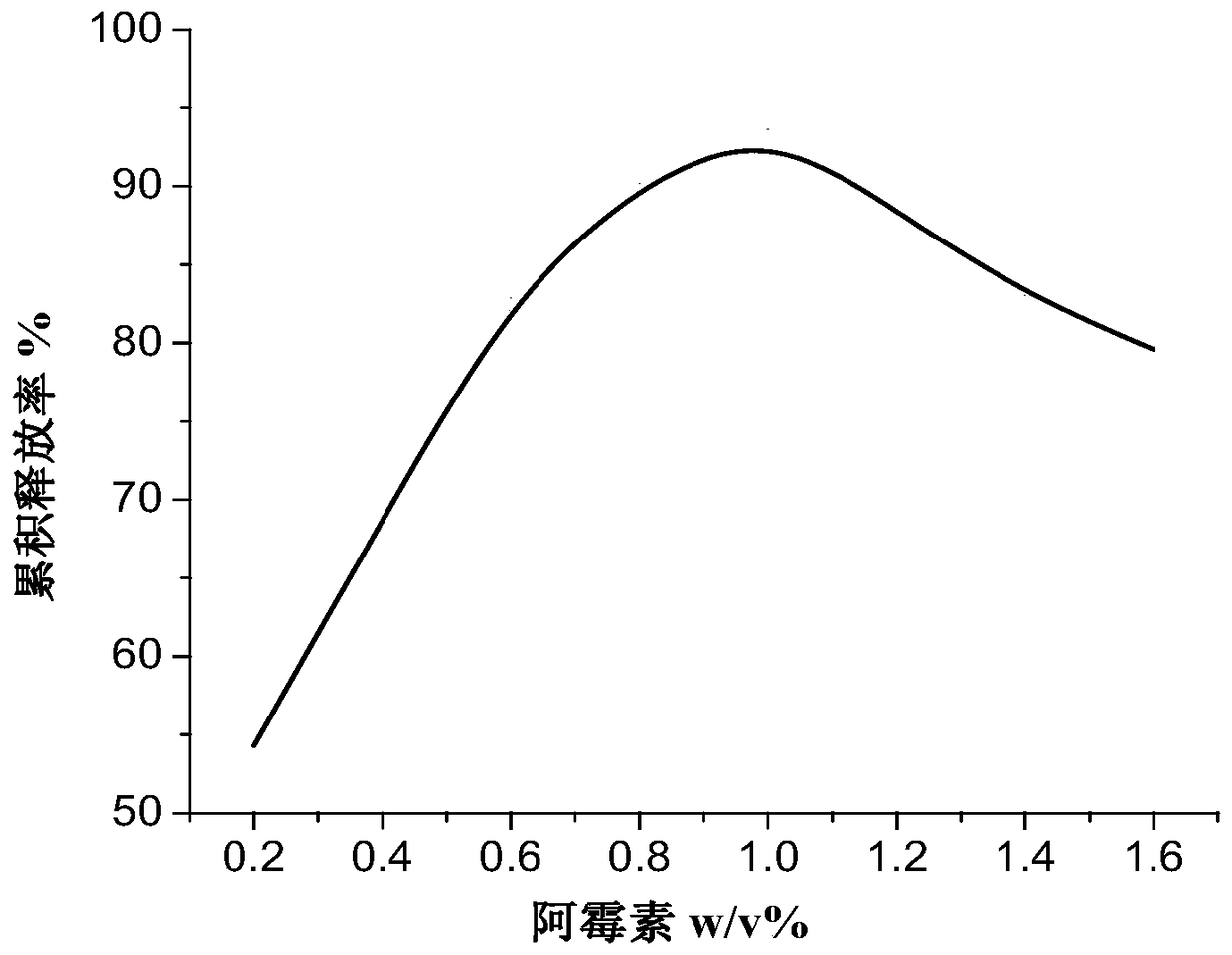
A drug-loading system of catechol-modified hyaluronic acid and its preparation method
A technology of hyaluronic acid and catechol is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, which can solve problems such as poor stability, achieve less side effects, and improve bioavailability. the effect of prolonging the retention time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Dissolve catechol-modified hyaluronic acid at a concentration of 1.5w / v% in a mixed solvent of dimethylformamide (DMF) / water, add doxorubicin to make the concentration 1.0w / v%, Stir at room temperature until completely dissolved, and let stand to obtain the spinning solution. Transfer the resulting spinning solution to the syringe, connect the spinning device, and adjust the parameters: the applied voltage during spinning is 22KV, the receiving distance is 15cm, and the injection speed is 0.5mL / h; the environmental parameters are set to temperature: 24°C , relative humidity: 45%, after a certain period of time, get nanofiber film, cut into 1×1cm 2 Size, ready to use after drying.
[0054] Cervical cancer cells (HeLa) were cultured on two cell slides, and the two cell slides were placed in a petri dish, and high-sugar DMEM culture solution (10% bovine serum albumin, 1% double antibody) was added, and the The nanofiber membrane obtained above was placed on a slide, cult...
Embodiment 2
[0056] Catechol-modified hyaluronic acid is dissolved in a mixed solvent of dimethylformamide (DMF) / water with a concentration of 1.5w / v%, and doxorubicin is added to make the concentration 1.0w / v%, and the temperature is kept at room temperature. Stir until completely dissolved, and let stand to obtain the spinning solution. Transfer the resulting spinning solution to the syringe, connect the spinning device, and adjust the parameters: the applied voltage during spinning is 22KV, the receiving distance is 15cm, and the injection speed is 0.5mL / h; the environmental parameters are set to temperature: 24°C , relative humidity: 45%, after a certain period of time, get nanofiber film, cut into 1×1cm 2 , after drying for later use.
[0057] Take the HeLa cell suspension in two centrifuge tubes, place the above-mentioned fiber membrane loaded with doxorubicin and the above-mentioned fiber membrane without doxorubicin in the two tubes respectively, take it out after 20 min, add high...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com