Method for detecting ferric ions through triphenylamine dye
A technology of triphenylamine and ferric iron, applied in the direction of material excitation analysis, fluorescence/phosphorescence, etc., can solve the problems of non-continuous detection, high detection cost, large sample consumption, etc., achieve low detection limit, low detection cost, high Effects of Selectivity and Sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Preparation of triphenylamine dyes
[0029] In a 100mL flask, add 1.3655g (5mmol) 4-(diphenylamine) benzaldehyde, 0.6608g (10mmol) malononitrile and an appropriate amount of triethylamine, and then add 50mL of absolute ethanol. After reacting at room temperature for 24 hours, the solvent was distilled off under reduced pressure, and the residual solid was purified with a silica gel column under the condition that the eluent composition was ethyl acetate:n-hexane=1:8 (v / v) to obtain 1.2521 g of a yellow solid. Yield 78%.
[0030] 2. Compound Characterization
[0031] 1 HNMR (400MHz, DMSO-d 6 )δ (ppm): 8.20 (s, 1H), 7.82 (d, J = 8.5Hz, 2H), 7.44 (t, J = 7.5Hz, 4H), 7.26 (dd, J = 19.7, 7.4Hz, 6H) ,6.83(d,J=8.5Hz,2H).
[0032] 13 CNMR (100MHz, DMSO-d 6 )δ (ppm): 159.30, 152.89, 144.70, 133.07, 130.13, 126.77, 126.24, 122.58, 117.41, 115.37, 114.42, 73.73;
[0033] IR(v -1 ,KBr):3057,2216,1610,1590,1568,1506,1440,1349,1317,1241,1189,830,763,697,524.
[0034] HR-MS...
Embodiment 2
[0039] Detection of Ferric Ion with Triphenylamine Dye
[0040] (1) Prepare a triphenylamine dye DMSO solution with a concentration of 10 mM, take 2 parts of 10 μL triphenylamine dye DMSO solution respectively, add distilled water to dilute to 10 mL respectively, and obtain 2 parts of triphenylamine dye aqueous solution;
[0041] (2) preparation concentration is the ferric chloride aqueous solution of 10mM, and concentration is the ferrous chloride aqueous solution of 60mM;
[0042] (3) In 2 parts of 10mL triphenylamine dye aqueous solution, add dropwise respectively 0.1mL of ferric chloride aqueous solution and ferrous chloride aqueous solution prepared in step (2), after mixing evenly, observe triphenylamines under 365nm light excitation Fluorescence changes in aqueous dye solutions.
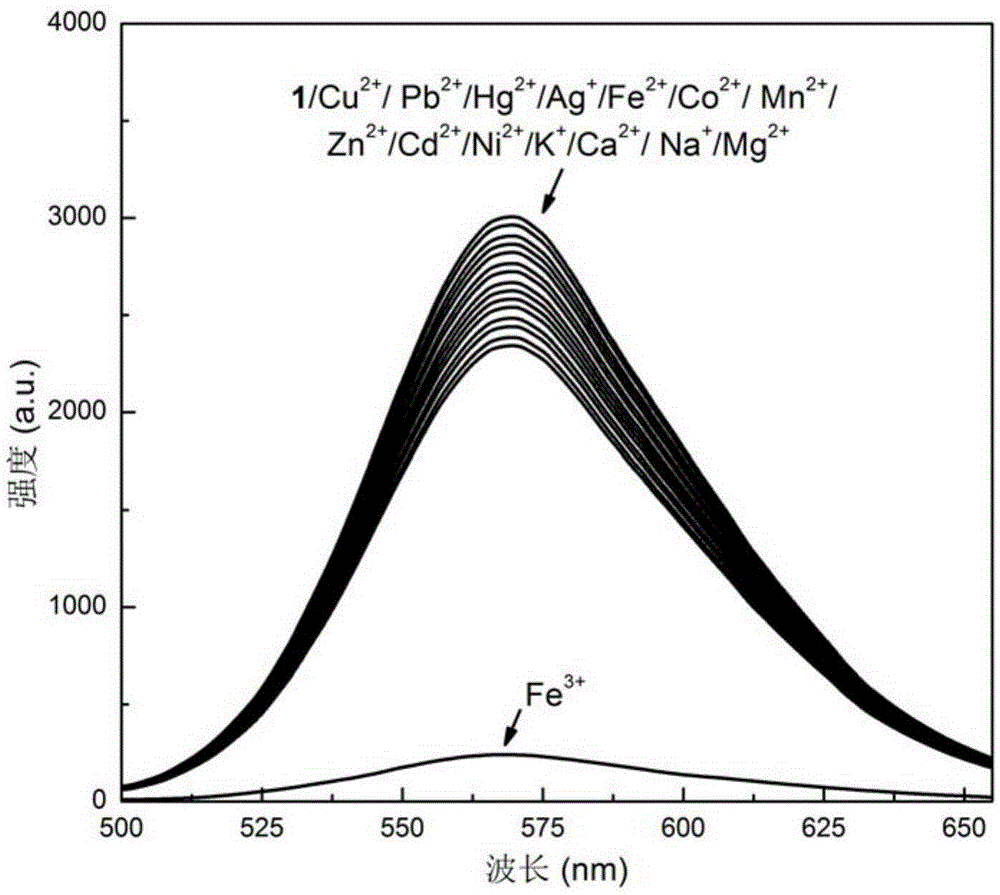
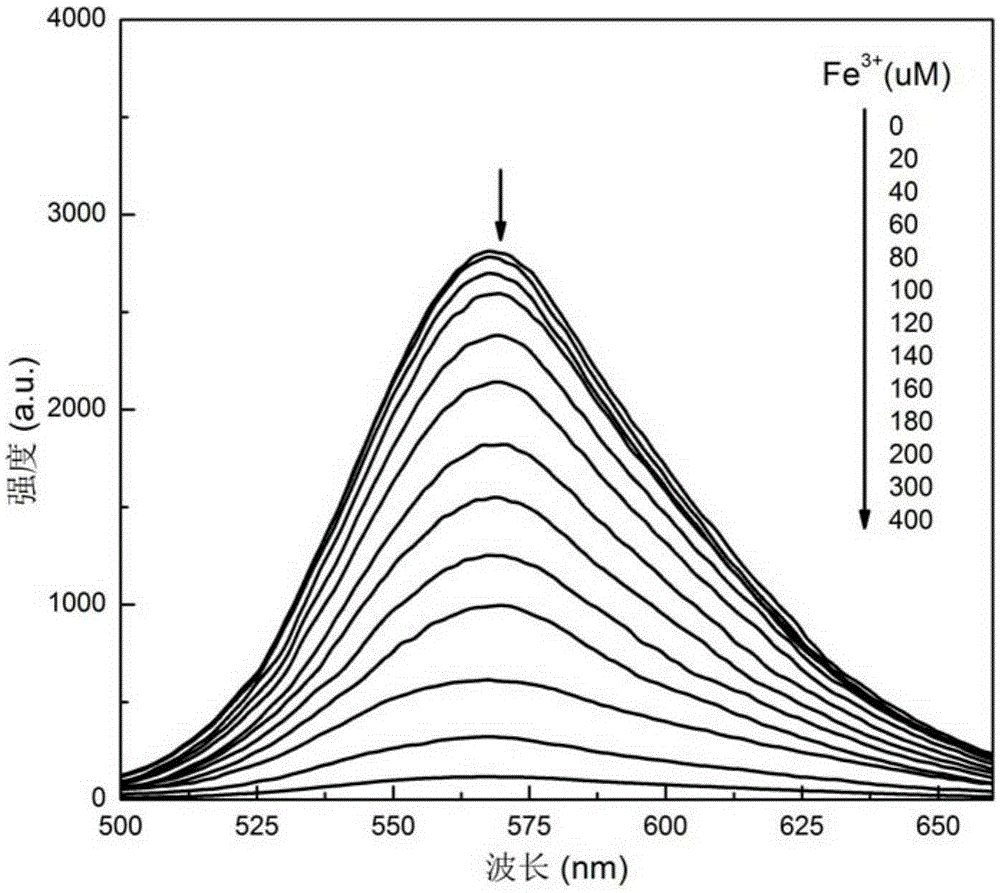
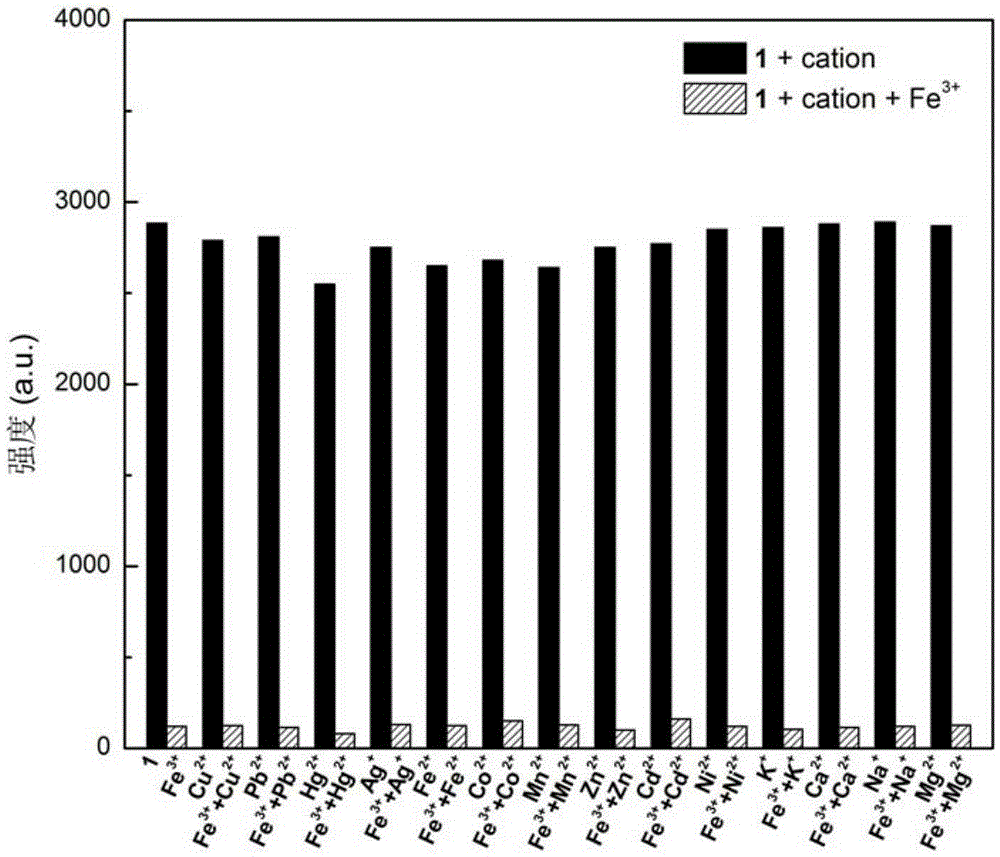
[0043] The results showed that after adding the ferric chloride aqueous solution, the fluorescence of the triphenylamine dye aqueous solution was quenched, while adding ferrous chloride, the ...
Embodiment 3
[0045] Detection of Ferric Ion with Triphenylamine Dye
[0046] (1) Prepare a triphenylamine dye THF solution with a concentration of 20 mM, take 2 parts of 10 μL triphenylamine dye THF solution respectively, add distilled water to dilute to 10 mL respectively, and obtain 2 parts of triphenylamine dye aqueous solution;
[0047] (2) preparation concentration is the ferric chloride aqueous solution of 15mM, and concentration is the copper sulfate aqueous solution of 60mM;
[0048] (3) In 2 parts of 10mL triphenylamine dye aqueous solution, add dropwise respectively 0.1mL of ferric chloride aqueous solution and copper sulfate aqueous solution prepared in step (2), after mixing evenly, observe the triphenylamine dye aqueous solution under 365nm light excitation changes in fluorescence.
[0049] The results showed that the fluorescence of the triphenylamine dye aqueous solution was quenched after adding the ferric chloride aqueous solution, and the fluorescence of the triphenylami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com