Magnetic resonance imaging contrast agent as well as preparation method and application thereof
A magnetic resonance imaging and contrast agent technology, which is applied in the directions of pharmaceutical formulations, nanotechnology for sensing, preparations for in vivo experiments, etc., can solve the problems of ferric oxide particle residues, etc., to reduce the concentration of iron ions, Rapid metabolism, easy long-term storage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of the above-mentioned contrast agent comprises the following steps:
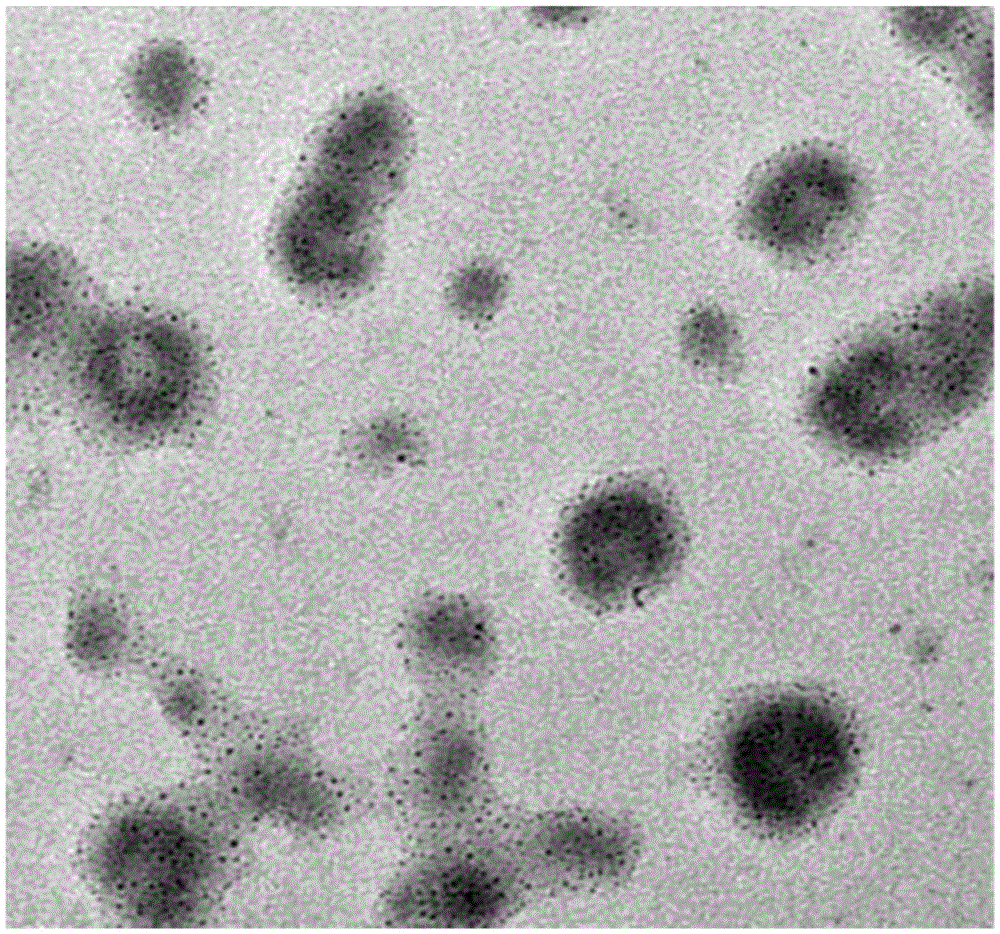
[0036] (1) The raw material powder is prepared as a 0.04% to 0.2% aqueous solution, and the free iron ions and small molecular compounds are removed by methods such as tangential flow ultrafiltration or dialysis; the raw material powder includes a mass fraction of 6% to 12% iron ferric oxide particles, 0.6% to 1.2% citric acid and 86.8% to 93.4% hydroxyethyl starch, the particle size of the iron ferric oxide particles is 60nm to 75nm, and the citric acid coating on the surface of the ferric oxide particles;
[0037] (2) Assuming that the quality of D-polylysine is 6% to 20% of the mass of the ferric oxide particles, add 0.04% to 0.2 times the volume of the aqueous solution in the step (1) to 0.01 to 0.05 times. % D-polylysine solution, so that D-polylysine is completely dispersed in the solution evenly, and is bound to the surface of the citric acid through ionic bonds to obta...
Embodiment 1
[0047] Example 1 The preparation method of D-polylysine encapsulating nano-scale iron ferric oxide particles
[0048] (1) take by weighing raw material powder (which contains 9.2wt% superparamagnetic nano iron ferric oxide particles, 0.8wt% citric acid, 88.5% average molecular weight is the hydroxyethyl starch of 130KDa, other part is in the raw material powder Impurities) 5 grams of solids are dissolved in 5.0 liters of pure water, placed in the storage container of the tangential flow ultrafiltration device (Pellicon2 device of Millipore Company), through the fiber membrane of ultrafiltration membrane block (5K molecular weight of Pellicon2 of Millipore Company) ) to carry out tangential flow ultrafiltration purification, the tangential flow velocity is set to the flow velocity (10 milliliters per minute) when the critical point of the tangential flow velocity linear differential pressure and saturation differential pressure, when the filter out container of the tangential fl...
Embodiment 2
[0053] Example 2 R-polylysine coated nano-scale ferric oxide lyophilized powder injection
[0054] After the liquid obtained in the step (2) of the above Example 1 is filtered through a 0.2 micron pore size filter, it is sub-packed in a glass bottle with a volume of 5 milliliters, and then freeze-dried in a vacuum. The powder obtained in the glass bottle is subjected to nitrogen gas Protected and sealed with a gland, that is, R-polylysine-coated nano-scale ferric oxide lyophilized powder injection. When using powder injection, inject 3.5 ml of clinical saline into the glass bottle, shake well and dissolve to prepare a solution. The recommended injection dose is 0.5 ml per 10 kg body weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com