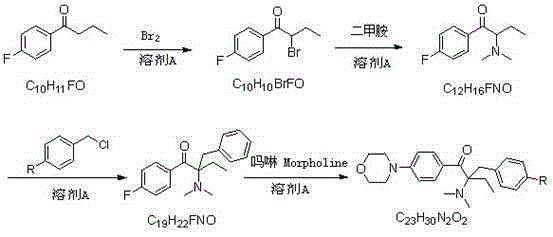
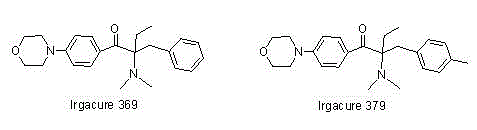
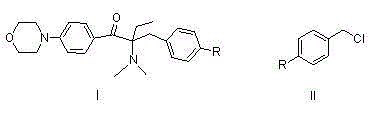Alpha-amino acetophenone photoinitiator preparation method
A technology of aminoacetophenone and photoinitiator, which is applied in the direction of organic chemistry, can solve the problems of unsuitable control of reaction temperature, increase of environmental pressure, and high cost of raw materials, so as to reduce the chance of cross-contamination, reduce the number of separations, and increase the reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Preparation of 1-p-fluorophenyl-1-butanone
[0045] In a 500ml four-neck flask, dissolve n-butyryl chloride (55.5g, 0.52mol) in dichloroethane (250ml), stir evenly, cool to about 0°C, and dissolve fluorobenzene (52.1, 0.57mol) in Add 100ml of dichloroethane dropwise to n-butyryl chloride solution, after dropping, keep warm for reaction, monitor the reaction by TLC or gas chromatography, after the reaction is complete, pour it into dilute ice hydrochloric acid solution, stir for 1h, let it stand, and separate the organic phase, washed with water and saturated sodium carbonate solution respectively, stripped, and finally rectified under reduced pressure to obtain 77.8g of the product (conditions for collecting fractions: 102-105°C / 9mmHg), with a yield of 90% and a purity of 99.0%. 1 HNMR (DMSO-d 6 ):d8.03(q,2H,J=9.0Hzand5.6Hz),7.31(t,2H,J=8.9Hz),2.97(t,2H,J=7.0Hz),1.65-1.55(m,2H) ,0.91(t,3H,J=7.3Hz).
Embodiment 2
[0046] Example 2: Preparation of 2-bromo-1-p-fluorophenyl-1-butanone
[0047] Take the 1-p-fluorophenyl-1-butanone (66.5g, 0.40mol) prepared according to the method of Example 1 and dissolve it in 140ml of toluene, stir well, add 6.7g of concentrated sulfuric acid dropwise under ice-water bath, and drop it for 0.5h , rise to room temperature, add bromine (51.1g, 0.32mol) previously dissolved in 70ml of toluene dropwise into the reaction system, drop for 1h, keep warm for reaction, monitor the reaction by TLC or liquid chromatography, after the reaction is complete, add 67ml of bromine water, stirred for 0.5h, allowed to stand for layers, separated the water phase, then extracted with 70ml of toluene, combined the toluene phase, washed with saturated sodium carbonate solution, and the resulting organic phase was 2-bromo-1-p-fluorophenyl-1-butanol The toluene solution of ketone, the liquid phase content is 96.8%, and it can be directly reacted in the next step without purificati...
Embodiment 3
[0048] Example 3: Preparation of 2-dimethylamino-1-p-fluorophenyl-1-butanone
[0049] The toluene solution of 2-bromo-1-p-fluorophenyl-1-butanone prepared in Example 2 was added to a 1L four-necked flask, and 90g of 40% dimethylamine aqueous solution (36g, 0.8mol) and sodium carbonate were added (21.2g, 0.2mol), stirring the reaction at room temperature, monitoring the reaction by TLC or liquid chromatography, after the reaction was complete, the layers were left to stand, the organic phase was separated, and then washed with water to obtain 2-dimethylamino-1-p-fluorophenyl- The toluene solution of 1-butanone has a liquid phase content of 96.5%, and continues to the next step without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com