Organic white light material with thermal activation delay and aggregation-induced emission performance and synthetic method and application thereof
A technology of aggregation-induced luminescence and thermal activation delay, applied in the field of OLED devices, can solve the problems of difficult control of host-guest ratio, complicated preparation process, etc., to overcome aggregation luminescence quenching, simple synthesis method and purification process, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
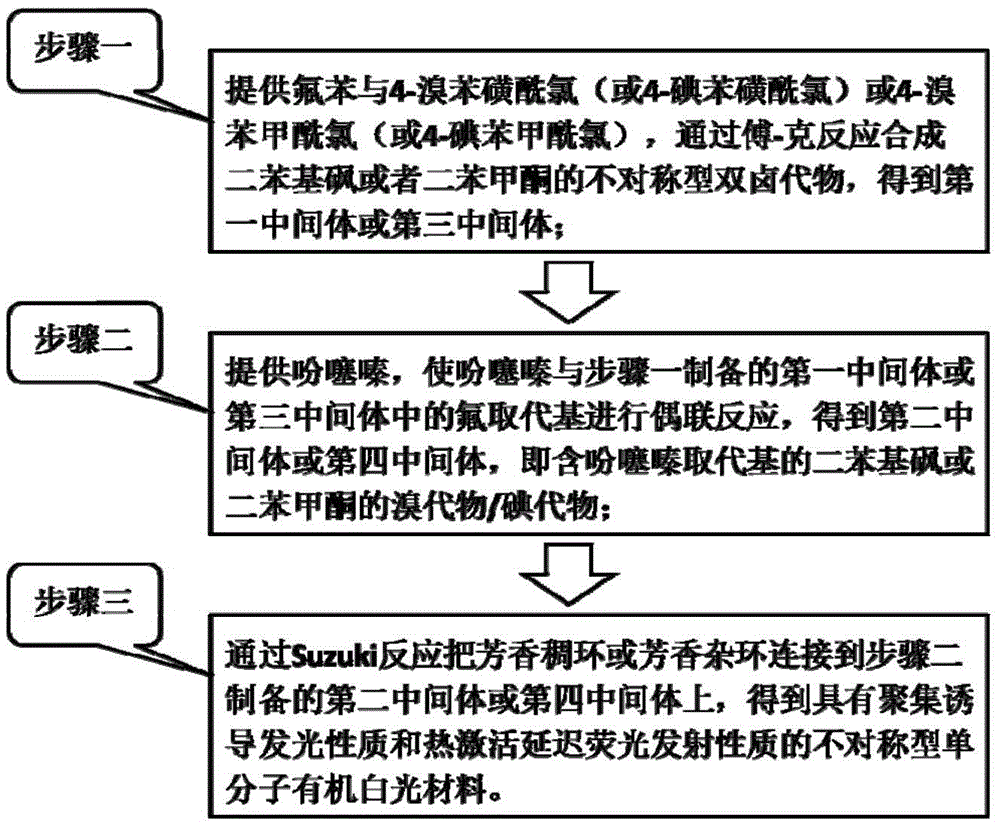
[0042] The synthesis method of the organic white light material of the present invention, such as figure 1 shown, including the following steps:
[0043] Step one: provide fluorobenzene and 4-bromobenzenesulfonyl chloride (or 4-iodobenzenesulfonyl chloride) or 4-bromobenzoyl chloride (or 4-iodobenzoyl chloride), take dichloromethane (DCM) as solvent in three Under the catalysis of ferric chloride, fluorobenzene is connected to 4-bromobenzenesulfonyl (or 4-iodobenzenesulfonyl) or 4-bromobenzoyl (or 4-iodobenzoyl) by Friedel-Crafts reaction, and synthesized to obtain first intermediate or third intermediate.
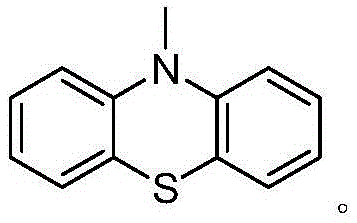
[0044] Step 2: Provide phenothiazine, use N,N-dimethylformamide (DMF) as a solvent to react with the first intermediate or the third intermediate under the action of potassium tert-butoxide, and synthesize the corresponding second intermediate or the fourth intermediate, Ar with a phenothiazine substituent 1 The halide; the halogen element of the halide is bromine or io...
Embodiment 1
[0048] Example 1: Synthesis of 10-(4-(4-(9-phenylanthracenyl-10-yl)benzenesulfonyl)phenyl)-10H-phenothiazine
[0049] (1) Synthesis of the first intermediate 1-(4-fluorobenzenesulfonyl)-4-bromobenzene, the synthetic route is as follows:
[0050]
[0051] Add 4-bromobenzenesulfonyl chloride (5.00g, 19.69mmol) and fluorobenzene (2.84g, 29.54mmol) into a 250mL three-necked flask, dissolve in 20mL of dichloromethane, add ferric chloride (6.33g, 39.38mmol) Afterwards, the reaction solution was heated to 40° C. and stirred for 6 h. The reaction solution was cooled to room temperature, and 30 mL of dichloromethane and 50 mL of 1M dilute hydrochloric acid were slowly added successively and stirred for 10 min. layer, and dried with anhydrous sodium sulfate, suction filtered, and the obtained filtrate was spin-dried with the solvent by a rotary evaporator, and the remaining solid was vacuum-dried to obtain 5.54 g of a white powder, with a yield of 90%.
[0052] (2) Synthesizing the...
Embodiment 2
[0058] Example 2: Synthesis of 10-(4-((4-(dibenzothiophen-4-yl)phenyl)sulfonyl)phenyl)-10H-phenothiazine
[0059] Synthesize the target product 10-(4-((4-(dibenzothiophen-4-yl)phenyl)sulfonyl)phenyl)-10H-phenothiazine, the synthetic route is as follows:
[0060]
[0061] The third intermediate 10-(4-(4-bromobenzenesulfonyl)phenyl)-10H-phenothiazine (0.50g, 1.01mmol) and dibenzothiophene-4-boronic acid (0.35g, 1.52mmol) Add it into a three-neck flask, dissolve it with 30mL tetrahydrofuran (THF), add 2MK 2 CO 3 Aqueous solution 1.5mL, argon and stirred for 30min, added 0.05gPd(PPh 3 ) 4 After that, it was heated to 75°C for 16h. After the reaction solution was cooled to room temperature, 20 mL of ethanol was added and spin-dried in a rotary evaporator under vacuum. The crude product was separated and purified by silica gel column chromatography using a mixed solution of n-hexane and dichloromethane with a volume ratio of 1:1 as the eluent. The obtained solid was a white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com