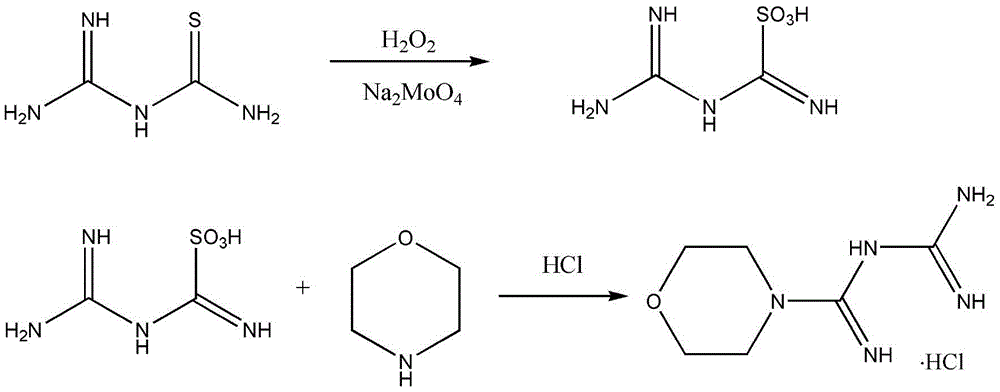
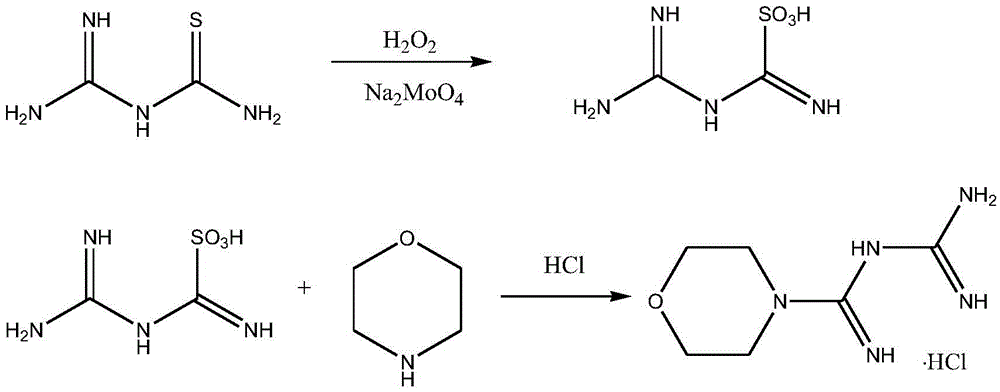
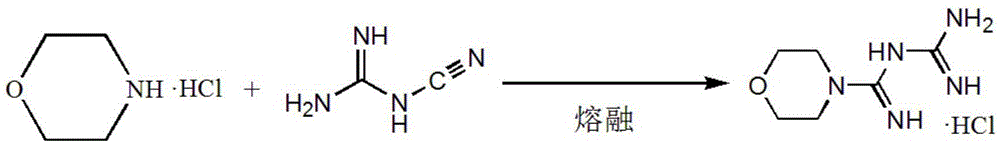Synthetic method of moroxydine hydrochlofide
A technology of morpholine amidine guanidine hydrochloride and a synthesis method, which is applied in the field of synthesis of morpholine amidine guanidine hydrochloride, can solve the problems of large heat release of hydrogen peroxide oxidation, low yield, difficult industrial application and the like, achieves low toxicity, lower reaction temperature, The effect of preventing self-aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) add diethylene glycol 2.1Kg, dicyandiamide 2.0Kg, ammonium chloride 1.4Kg successively in the reactor of 10L band mechanical stirrer, mass mass concentration is the sulfuric acid aqueous solution 0.5Kg of 30%, under sealed stirring state , the temperature was raised to 120°C at a rate of 3°C per minute, the pressure of the reactor was raised to 0.3MPa, and after 1 hour of reaction, the reaction temperature was naturally cooled to room temperature to obtain a white emulsion;
[0040](2) Transfer the white emulsion to a 50L glass reactor, add 15Kg of 95% ethanol, heat and reflux for 30 minutes to obtain a mixed solution, heat the mixed solution through a filter cartridge to a crystallization kettle, and cool the filtrate in a crystallization kettle After reaching room temperature, white crystals were precipitated, and after centrifugation, they were dried in a hot air oven at 80°C to obtain 5.2Kg of morpholine amididine hydrochloride, with a yield of 81% based on dicya...
Embodiment 2
[0042] (1) add diethylene glycol 3.1Kg, dicyandiamide 3.0Kg, ammonium chloride 2.0Kg successively in the reactor of 10L band mechanical stirrer, mass concentration is the aqueous solution 0.5Kg of 20% sulfuric acid, under sealed stirring state, Raise the temperature to 120°C at a rate of 3°C per minute, and raise the pressure of the reactor to 0.5MPa. After reacting for 1 hour, naturally cool the reaction temperature to room temperature to obtain a white milky product;
[0043] (2) Transfer the white emulsion to a 50L glass reaction kettle, add 28Kg of 95% ethanol, heat and reflux for 30 minutes to obtain a mixed solution, heat the mixed solution through a filter cartridge to a crystallization kettle, and cool the filtrate in a crystallization kettle After reaching room temperature, white crystals were precipitated, and after centrifugation, they were dried in a hot air oven at 85°C to obtain 6.2Kg of morpholine amididine hydrochloride, with a yield of 83% based on dicyandiamid...
Embodiment 3
[0045] (1) add diethylene glycol 2.6Kg, dicyandiamide 2.5Kg, ammonium chloride 1.6Kg successively in the reactor of 10L band mechanical stirrer, mass concentration is 0.5Kg of aqueous sulfuric acid solution of 20%, under sealed stirring state, Raise the temperature to 130°C at a rate of 5°C per minute, and raise the pressure of the reactor to 0.4MPa. After reacting for 1 hour, naturally cool the reaction temperature to room temperature to obtain a white milky product;
[0046] (2) Transfer the white emulsion to a 50L glass reaction kettle, add 20Kg of 95% ethanol, heat and reflux for 30 minutes to obtain a mixed solution, heat the mixed solution through a filter cartridge to a crystallization kettle, and cool the filtrate in a crystallization kettle After reaching room temperature, white crystals were precipitated. After centrifugation, they were dried in a hot air oven at 80° C. to obtain 5.3 kg of morpholine amididine hydrochloride, with a yield of 85% based on dicyandiamide....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com