Isophorone fluorescence probe, and preparation method and application thereof
A technology of fluorescent probe and isophorone, which is applied in the preparation of carboxylic acid nitrile, fluorescence/phosphorescence, and preparation of organic compounds, can solve the problems of not being able to measure cells and large detection errors, and achieve good biomembrane communication. Permeability, avoidance of interference, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
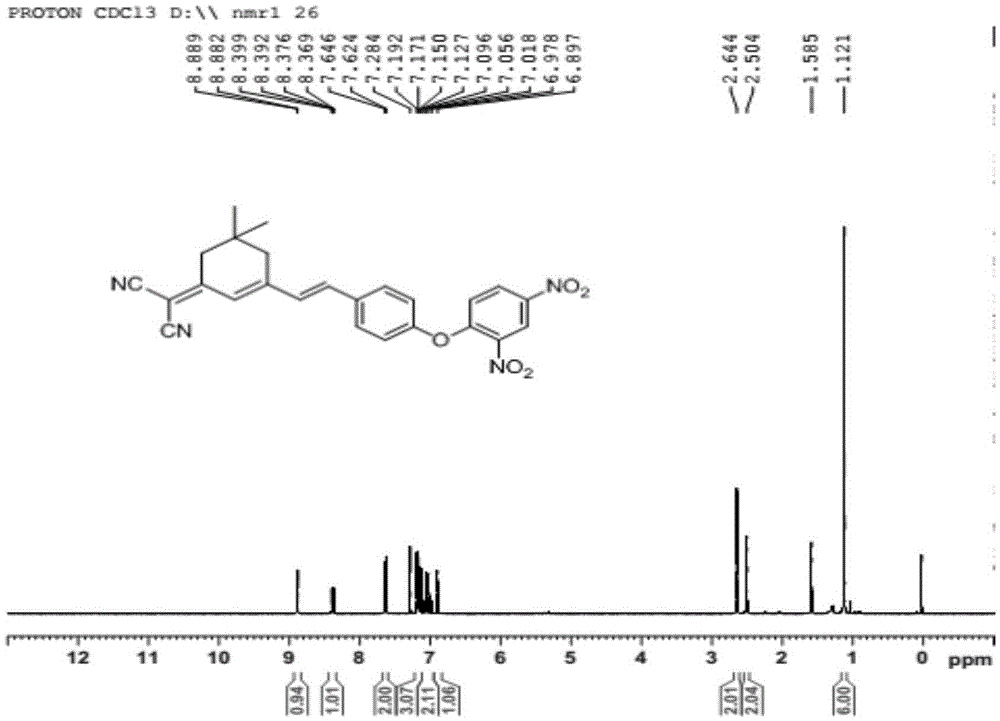
[0032] Embodiment 1: the preparation of a kind of isophorone fluorescent probe comprises the following steps:
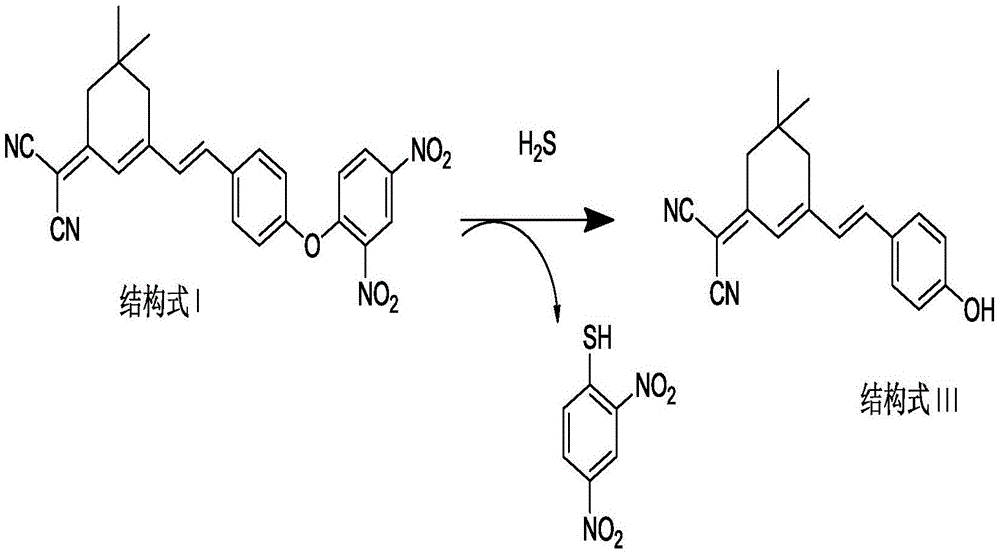
[0033] (1) Preparation of the compound of formula II: under the protection of inert gas, add isophorone (1.0mmol), malononitrile (1.0-4.0mmol), pyridine (0.1mmol-0.4mmol) into the reaction flask, and ethanol as the solvent , heated to reflux for 18-24h, cooled to room temperature after the reaction, the reaction solution was purified by silica gel column chromatography, the eluent was V petroleum ether: V ethyl acetate = 2-5:1, and the pure product of the compound of formula II was obtained;
[0034] (2) Preparation of the compound of formula III: under the protection of inert gas, the compound of formula II (1.0mmol), p-hydroxybenzaldehyde (1.0~4.0mmol) and pyridine (0.1~0.4mmol) are added in the reaction flask, ethanol is the solvent, Heating and reflux for 4-18 hours, after the reaction was completed, lowered to room temperature, concentrated, added deionized wate...
Embodiment 2
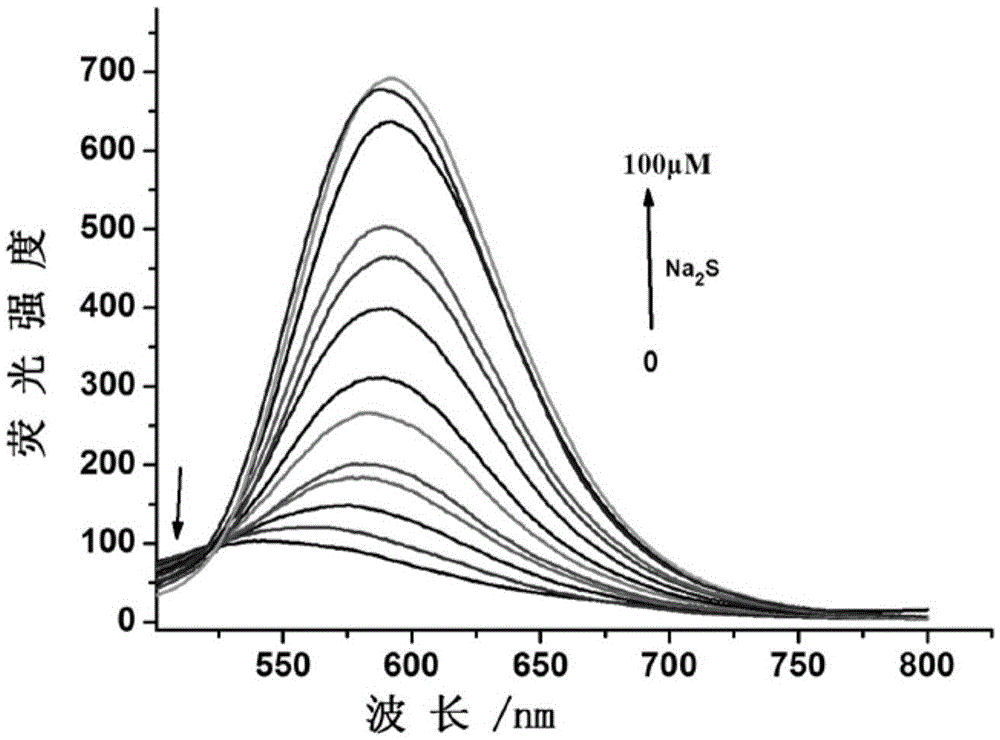
[0043] The water from the Kuihe River in Xuzhou City and the river flowing in the campus of Xuzhou Medical College were taken as representative water samples to further study the reliability of the probe molecules of the present invention for the detection of water samples. First filter the water samples to be tested, and add different concentrations of HS - solution (0, 1, 10, 60, 100 μM), add the fluorescent probe represented by formula I to make the final concentration 10 μM, and incubate at 37° C. for 1 h. Use a fluorescence spectrophotometer to detect the change in the response value of the reaction solution at 592nm, and the test results are as follows: Figure 7 shown. The fluorescence intensity response value of each sample is consistent with that of the standard sample. This result shows that the fluorescent probe molecule of the present invention can be used to quantitatively detect the concentration of hydrogen sulfide in the water environment.
Embodiment 3
[0044] Embodiment 3: Fluorescent probe molecule of the present invention detects hydrogen sulfide in food
[0045] Taking pork homogenate as a representative sample, the reliability of the probe molecule of the present invention for the detection of hydrogen sulfide in food was further studied. Take 9 parts of freshly purchased pork, each 3 parts as a group, put them in a constant temperature and humidity incubator to accelerate spoilage, take out 10 g of the required sample amount every 12 hours, homogenize and take the supernatant, add different concentrations of HS - solution (0, 1, 10, 60 μM), add the fluorescent probe shown in formula I to make the final concentration 10 μM, incubate at 37°C for 1h, and detect the response value change of the reaction solution at 592nm with a fluorescence spectrophotometer. Test results such as Figure 8 shown. The fluorescence intensity response value of each sample was basically the same as that of the standard sample, and the concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com