A kind of styrene acid compound, its composition and its application
A technology of styrene acid and composition, which is applied in the field of styrene acid compounds, can solve the problems of diabetic eye disease research, increase the risk of drug use, increase the frequency of drug administration, etc., achieve improvement of optic neuropathy or choroidal lesions, prolong the action time, improve The effect of permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
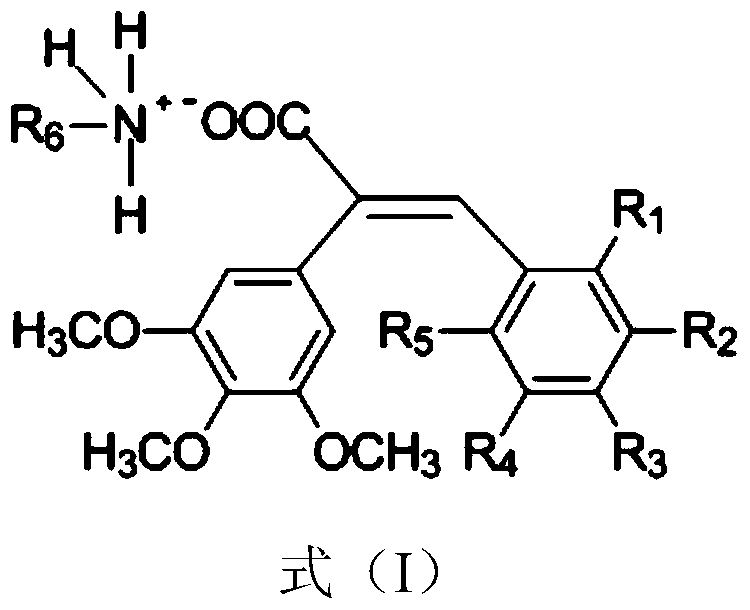
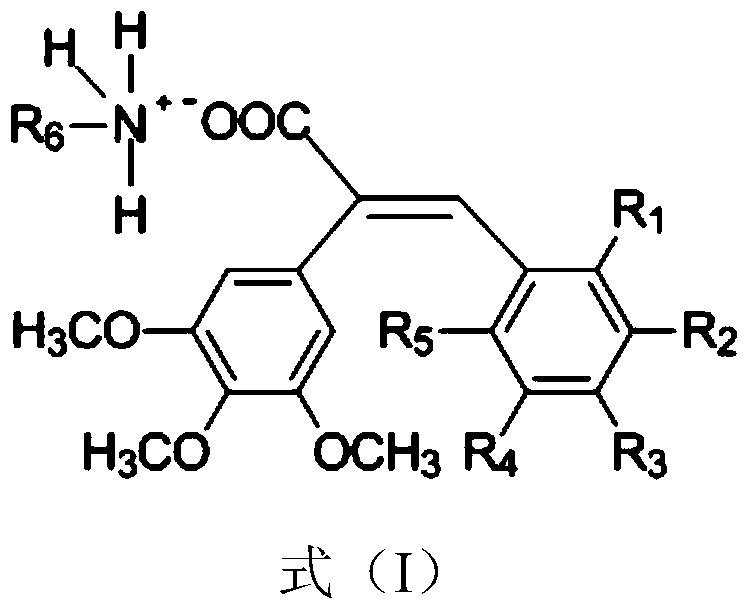
[0043] Example 1: (E)-3-(3'-Hydroxy-4'-methoxyphenyl)-2-(3", 4", 5"-trimethoxyphenyl) 2-n-butylammonium acrylate salt preparation
[0044] Mix 3,4,5-trimethoxyphenylacetic acid (610mmol), 3-hydroxy-4 methoxybenzaldehyde (724mmol), acetic anhydride (200mmol) and triethylamine (717mmol), stir and reflux at 150°C for 2.5 h, distill the solvent off under reduced pressure, add a sufficient amount of 1N hydrochloric acid (about 300ml), stir at room temperature overnight, filter the resulting solid and recrystallize it with ethanol to obtain (E)-3-(3'-hydroxy-4'-methoxy Phenylphenyl)-2-(3”, 4”, 5”-trimethoxyphenyl) 2-acrylic acid. Dissolve the obtained phenylacrylic acid derivative in acetone (about 30ml), and drop it under the protection of argon at 0℃ Add an equivalent amount of n-butylamine acetone solution, heat up to 20°C and react for 1h, concentrate under reduced pressure to remove the solvent, add ethyl acetate (about 30ml), heat to 80°C for reflux, then cool to 0°C, filter ...
Embodiment 2
[0046] Example 2: Effects of phenylacrylic acid compounds on neovascularization in rats with diabetic retinopathy
[0047] Experimental animals: 180 healthy male Wistar rats aged 6 weeks, weighing 180-220 g. The feeding conditions are stored at room temperature 18-25° C., air circulation, relative humidity 55-75%, and 12-hour light maintenance.
[0048] Modeling method: above normal adult male Wistar rats, the lenses of both eyes are completely transparent without any turbidity. After feeding for one week, the body weight was measured after fasting for 12 hours. Streptozotocin (purchased from Laibo Bio-Experimental Materials Research Institute) was dissolved in 0.1ml / L, pH4.2 in citric acid buffer solution to prepare 1% streptozotocin solution, the experimental group was 60mg / L One-time intraperitoneal injection of the above solution with a body weight of kg, followed by adequate drinking water and food, induced diabetic retinopathy for modeling. The normal control group wa...
Embodiment 3
[0072] Example 3: Study on drug action time based on the effect of phenylacrylic acid compounds on the neovascularization of rat diabetic retinopathy
[0073] The test was repeated according to the method of Example 2, only the administration method was replaced by each group adopting administration once every two days. The normal control group and the experimental control group were intraocularly injected with 1.0 μL of phosphate buffer solution every two days, and the treatment groups A-F were intraocularly injected with 1.0 μL of drug solution every two days, with a drug concentration of 1.0 μg / μL.
[0074] test results:
[0075] 1) Immunization result:
[0076] In the normal control group, there was no positive expression of vascular endothelial growth factor (ie, VEGF) and fibroblast production chair (ie, bFGF), and the retinal cells were arranged neatly, and the cell morphology was normal; in the test control group, VEGF and bFGF were strongly positive in the whole laye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com