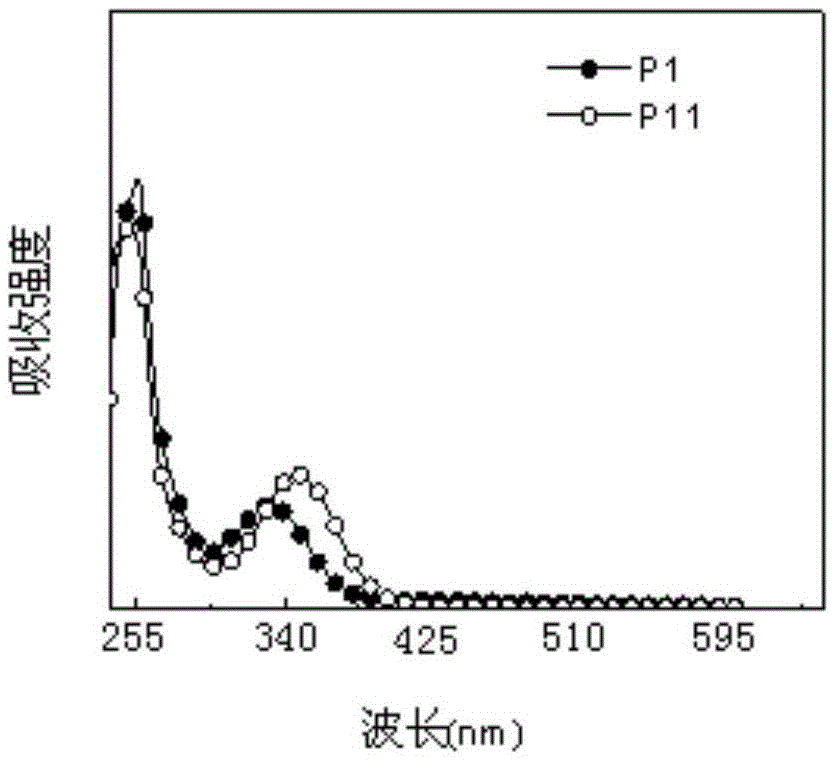
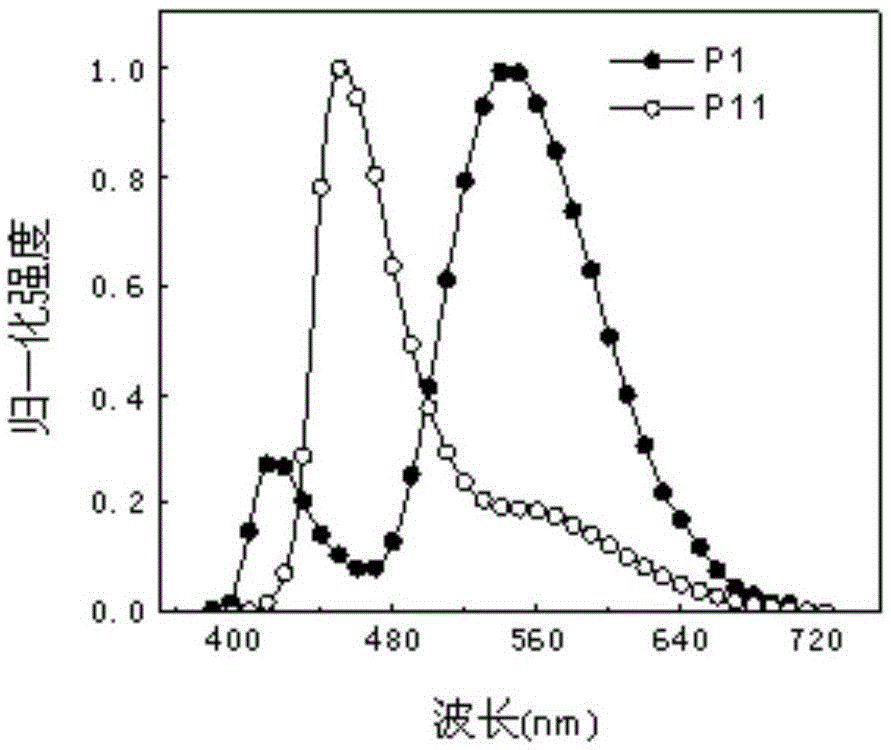
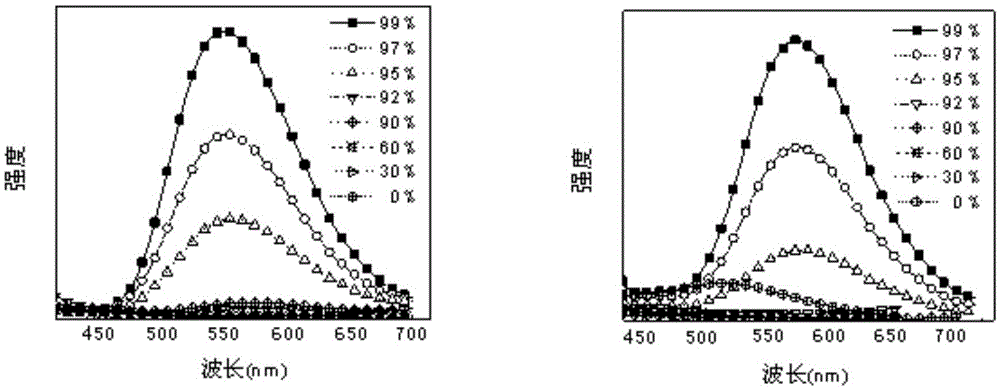
Multi-stimulus-response organic small-molecular luminescent material, and preparation and application thereof
A technology of light-emitting materials and small molecules, applied in the fields of light-emitting materials, organic chemistry, material excitation analysis, etc., which can solve the problems of lack of correlation between stimulus-response light-emitting compounds, scarcity of stimulus-response light-emitting materials, and irregular design of light-emitting compounds. , to achieve a simple, feasible and effective design strategy, improve sensitivity, and determine the effect of molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Prepare synthetic raw material 4-bromobenzophenone;
[0036] (2) Preparation of multi-stimuli-responsive organic small molecule luminescent material P1, the specific synthesis route is shown in the following formula:
[0037]
[0038] The specific implementation steps are:
[0039] Under nitrogen protection, add 100ml of toluene, 1g of 4-bromobenzophenone (3.85mmol), 0.92g of phenothiazine (4.62mmol, 1.2equ) into the three-necked flask, and add 0.58g of sodium tert-butyl alkoxide under stirring , then add 38.5mgPd(OAc) 2 (palladium acetate), tri-tert-butylphosphine, react overnight at 90°C. Cool down, extract the organic phase with dichloromethane, spin dry, and pass through the column. 1.17 g of a yellow solid product was obtained, with a yield of 80%. Molecular formula: C 25 h 17 NOS; M / Z=379.10 Theoretical: 379.10 (100.0%), 380.11 (27.0%), 381.10 (4.5%), 381.11 (2.7%), 382.10 (1.2%); Elemental analysis: C, 79.13; H, 4.52 ; N, 3.69; O, 4.22; S, 8.45.
Embodiment 2
[0041] (1) Prepare synthetic raw material 4-bromobenzophenone;
[0042] (2) Preparation of multi-stimuli-responsive organic small molecule luminescent material P2, the specific synthesis route is shown in the following formula:
[0043]
[0044] The specific implementation steps are:
[0045] Under nitrogen protection, add 100ml toluene, 1g4-bromobenzophenone (3.85mmol), 1.5g intermediate 7 (N-p-phenylboronic acid phenothiazine) to the three-necked flask, and add 2M potassium carbonate aqueous solution under stirring 25mL and 25mL ethanol, then add 200mg tetrakis (triphenylphosphine) palladium {Pd (PPh 3 ) 4}, react overnight at 90°C. After the reaction, the temperature was lowered, and the organic phase was extracted with dichloromethane, spin-dried, and passed through the column. 1.44 g of a yellow solid product was obtained, with a yield of 84%. Molecular formula: C 31 h 21 NOS; M / Z=379.10 Theoretical value: 517.08 (100.0%), 518.09 (33.5%), 519.09 (5.4%), 519.08 (...
Embodiment 3
[0051] (1) Preparation of intermediate 1, the specific synthetic route is shown in the following formula:
[0052]
[0053] The specific implementation steps are:
[0054] Under a nitrogen atmosphere, add 1.26g (8mmol) of 2-bromopyridine to a 100ml two-necked flask and dissolve it with 30ml THF; add 2.21g p-bromobenzaldehyde (12mmol, 1.5equ) to a 50ml eggplant-shaped flask and dissolve it with 20ml THF . The 2-bromopyridine tetrahydrofuran solution was cooled to -110°C with liquid nitrogen, argon was blown, and after 30 minutes of heat preservation, 3.2ml of 2.5M n-butyllithium (8mmol, 1equ) was slowly added dropwise. After dripping, react for one hour. After p-bromobenzaldehyde tetrahydrofuran was injected into the reaction system, the reaction was stirred at room temperature for three hours. After the reaction was completed, the tetrahydrofuran was evaporated to dryness, extracted three times with dichloromethane and water, and the yellow oil was obtained after the dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com