Quality control method of colloidal bismuth pectin pharmaceutical composition
A quality control method and colloidal bismuth pectin technology are applied in the field of improving the quality of a colloidal bismuth pectin pharmaceutical composition, which can solve the problems of poor product controllability, research, inability to further improve the curative effect of colloidal bismuth pectin, and the like. Safety, avoiding toxic and side effects, improving drug stability and efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0044] 1. Preparation of colloidal bismuth pectin
[0045] (1) Add 166.67g of purified water into the reaction flask, add 29.22g of bismuth nitrate; add about 23.78g of 40% potassium hydroxide solution, adjust the pH to 6-8, and make it hydrolyze completely (that is, retest the pH to be constant) Finally, filter to get the filter cake bismuth hydroxide; then put 40.56g of purified water and 17.89g of sorbitol into the beaker, and then add the filter cake bismuth hydroxide after the sorbitol dissolves, and add 40% potassium hydroxide after stirring About 50g of the solution, stir to make it fully dissolve, and obtain the bismuth salt solution for later use;
[0046] In (2), add the bismuth salt solution prepared in step (1) into the reaction flask, stir, add 49ml of purified water, add pectin soft material at room temperature, after the addition is completed, heat up to about 40°C and stir, keep the temperature for 0.5 hours, and then Add 105g of purified water, control the te...
experiment example
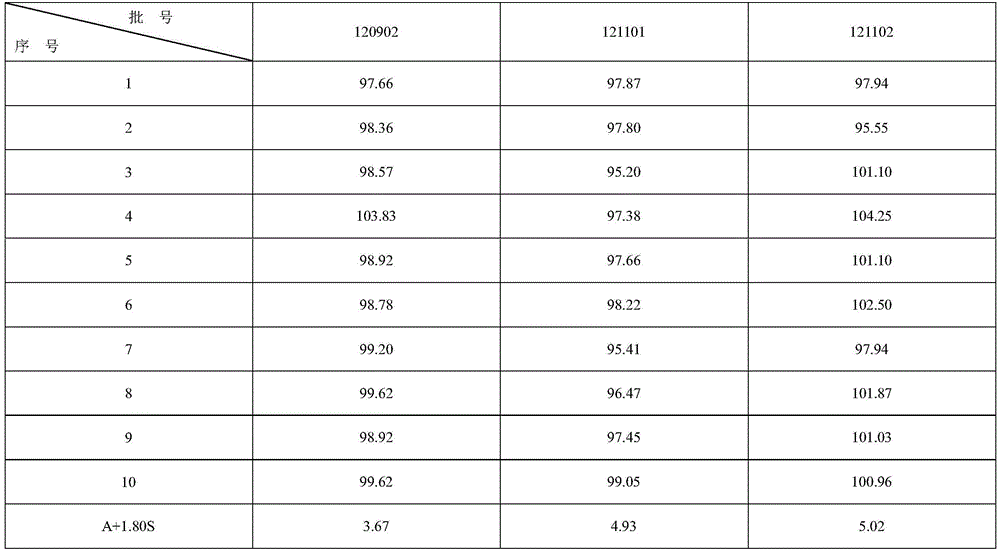
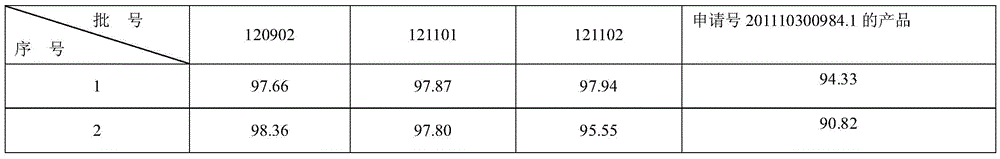
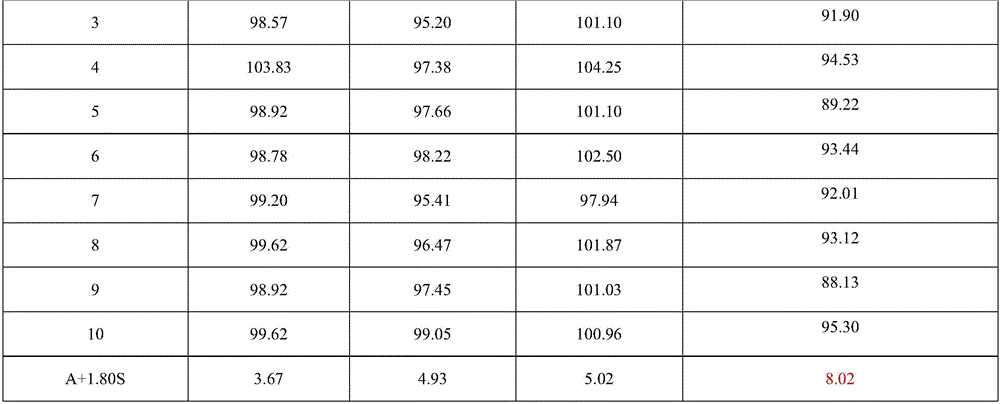
[0074] 1. Determination of content uniformity of colloidal bismuth pectin pharmaceutical composition
[0075] In the present invention, the content uniformity is according to the Chinese Pharmacopoeia 2010 edition two appendix XE content uniformity method: research and detect with reference to the method under the content determination item.
[0076] Assay method is: get 3 batches of colloidal pectin bismuth dry suspension product prepared according to the embodiment of the present invention 2, each 10 groups, respectively put in the conical flask of 500ml, add nitric acid solution 10ml, concentrated nitric acid and water in the nitric acid solution The volume ratio is 1:2, heat to dissolve, add 300ml of water and 4 drops of xylenol orange indicator solution, and titrate with disodium edetate titration solution (0.05mol / L) until the solution turns yellow. Every 1ml of disodium edetate titration solution (0.05mol / L) is equivalent to 10.45mg of bismuth (Bi). The results are sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com