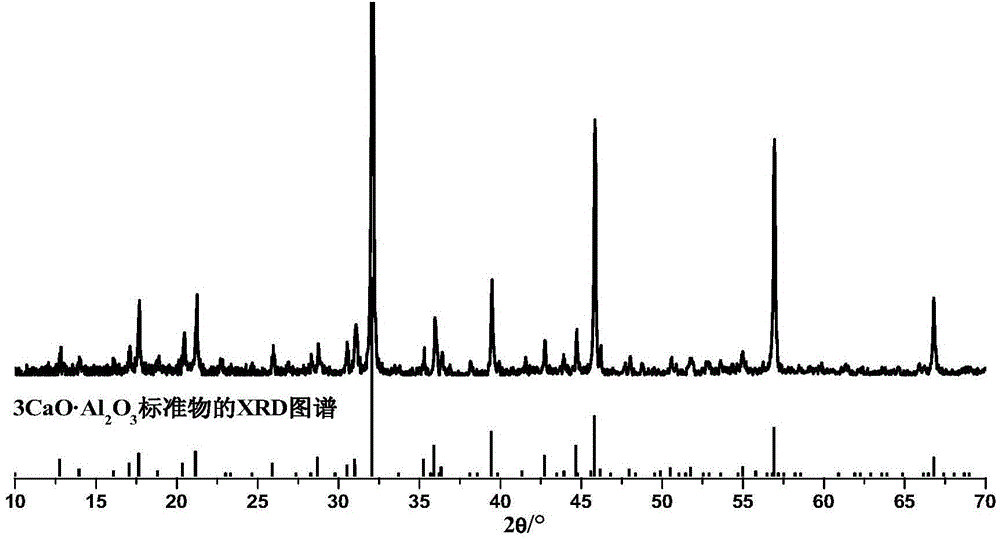
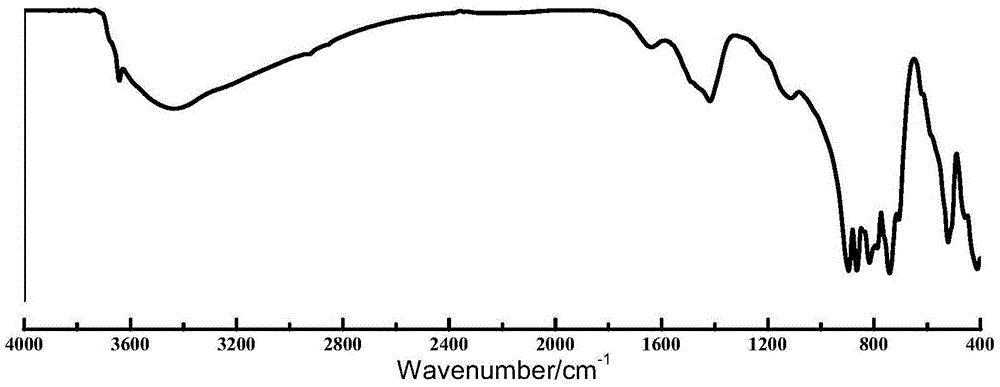
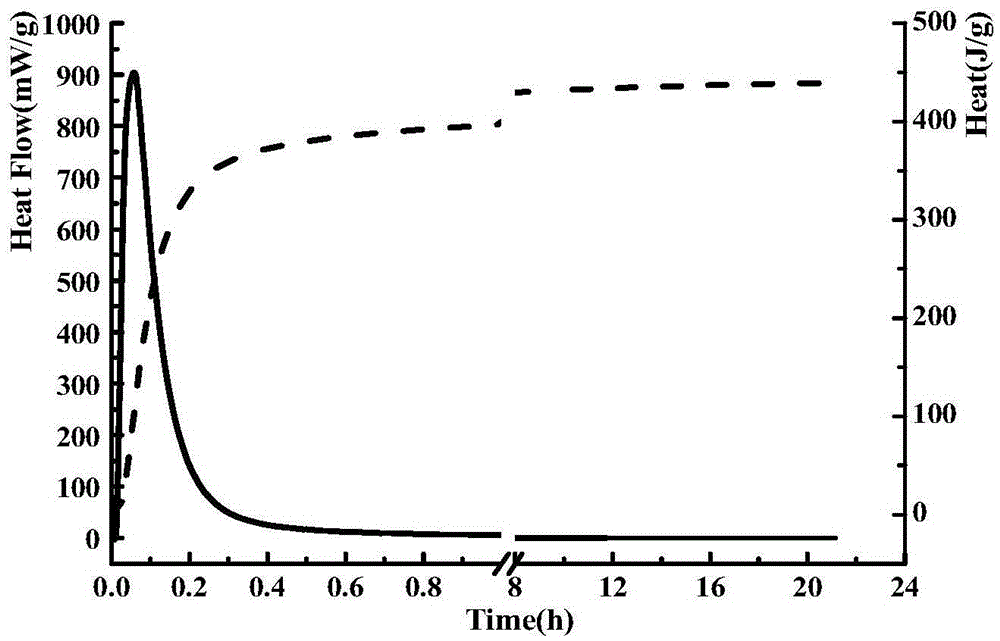
Method for preparing tricalcium aluminate by aid of spark plasma sintering techniques
A discharge plasma, tricalcium aluminate technology, applied in the preparation of calcium aluminate, alkaline earth metal aluminate/alumina/aluminum hydroxide, sustainable manufacturing/processing, etc., can solve the impact of formation, reduce calcination temperature, time It can reduce energy consumption and cost, irregular particle morphology and consistent chemical composition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Objective: To prepare 20.00g of tricalcium aluminate.
[0041] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content is 37.73%. According to the mass of tricalcium aluminate to be prepared is 20.00g, the mass of CaO in the sample after sintering is calculated to be 20.00×62.27%=12.45g.
[0042] According to the conservation of Ca element mass, the analytically pure calcium carbonate CaCO is calculated as 1.7847 times the mass of CaO 3 The required amount is 12.45×1.7847=22.22g;
[0043] Analytical Pure Calcium Carbonate CaCO 3 0.3396 times the mass to calculate the required analytically pure γ-phase alumina γ-Al 2 o 3 The mass of is 22.22×0.3396=7.55g.
[0044] Calculation and analysis of pure calcium carbonate CaCO 3 and analytically pure γ-phase activated alumina γ-Al 2 o 3 The total mass of the powder is 22.22+7.55=29.77g, and the required ball mass is calculated...
Embodiment 2
[0049] Purpose: To prepare 15.00 g of tricalcium aluminate.
[0050] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content is 37.73%. According to the mass of tricalcium aluminate to be prepared is 15.00g, the mass of CaO in the sample after sintering is calculated to be 15.00×62.27%=9.34g.
[0051] According to the conservation of Ca element mass, the analytically pure calcium carbonate CaCO is calculated as 1.7847 times the mass of CaO 3 The required amount is 9.34×1.7847=16.67g;
[0052] Analytical Pure Calcium Carbonate CaCO 3 0.3396 times the mass to calculate the required analytically pure γ-phase alumina γ-Al 2 o 3 The mass of is 16.67×0.3396=5.66g.
[0053] Calculation and analysis of pure calcium carbonate CaCO 3 and analytically pure γ-phase activated alumina γ-Al 2 o 3 The total mass of 16.67+5.66=22.33g, according to 4 times of the total mass of the powder mate...
Embodiment 3
[0058] Purpose: To prepare 10.00 g of tricalcium aluminate.
[0059] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content is 37.73%. According to the mass of tricalcium aluminate to be prepared is 10.00g, the mass of CaO in the sample after sintering is calculated to be 10.00×62.27%=6.23g.
[0060] According to the conservation of Ca element mass, the analytically pure calcium carbonate CaCO is calculated as 1.7847 times the mass of CaO 3 The required amount is 6.23×1.7847=11.12g;
[0061] Analytical Pure Calcium Carbonate CaCO 3 0.3396 times the mass to calculate the required analytically pure γ-phase alumina γ-Al 2 o 3 The mass of is 11.12×0.3396=3.78g.
[0062] Calculation and analysis of pure calcium carbonate CaCO 3 and analytically pure γ-phase activated alumina γ-Al 2 o 3 The total mass of 11.12+3.78=14.90g, according to 4 times the total mass of the powder materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com