Preparation method and antitumor effect of ginsenoside F1
A technology of ginsenosides and ginseng flowers, which is applied in the field of medicine, can solve the problems of unprepared and purified ginsenosides and low purity, and achieve the effects of no toxic side effects, drug dependence, high purity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The object of the present invention is to provide a kind of active ingredient that prepares a kind of anti-tumor effect-ginsenoside F from ginseng flower methanol extract 1 extraction and purification method. Specific steps are as follows:
[0047] (1) Preparation of Panax ginseng saponins
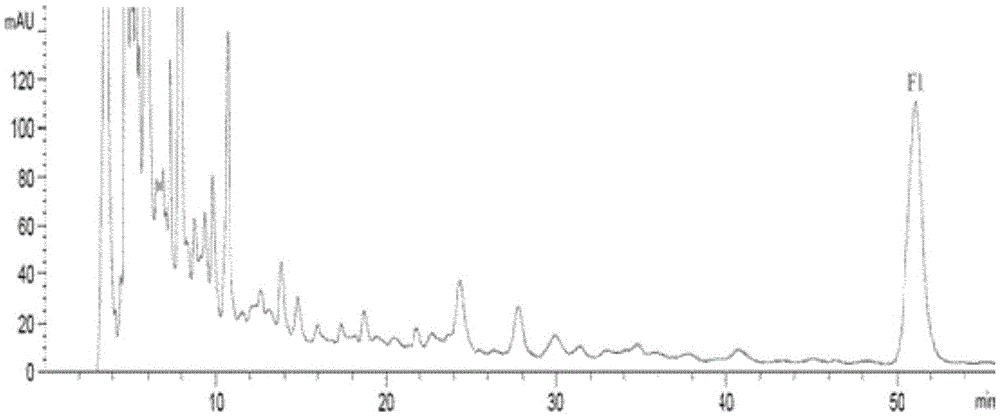
[0048] Take 4.02kg of freshly dried ginseng flowers, crush them, add 16L of methanol solution, heat and reflux for extraction, 2 hours each time, extract 3 times in total, combine the extracts, recover the solvent under reduced pressure to dryness, and obtain 1130g of methanol extract. Add 500 mL of water to the methanol extract, disperse and dissolve, extract with cyclohexane and ethyl acetate in sequence until the color of the solution becomes significantly lighter, recover and concentrate the ethyl acetate extract under reduced pressure to obtain 355 g of the ethyl acetate extraction fraction. Ginsenoside F 1 of ginseng flower saponins ( figure 1 ), the yield is about 8.8%. ...
Embodiment 2
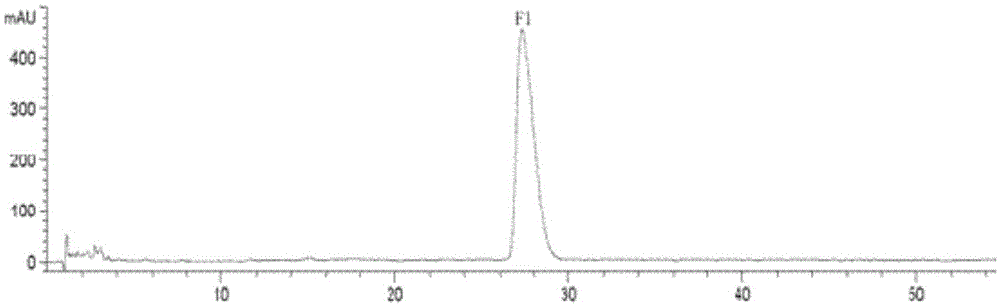
[0060] (1) Purity test
[0061] Ginsenoside F purified by column chromatography was purified by high performance liquid chromatography (HPLC). 1 Detected, its purity is 95.17% ( figure 2 ). Chromatographic conditions: chromatographic column, ZorbaxEclipseXDBC-18 (5μm; 4.6mmi.d. × 250mm); mobile phase, methanol: water volume ratio of 60:40; flow rate, 1.0mL / min; detection wavelength, 203nm; injection volume, 20 μL. Under this chromatographic condition, ginsenoside F 1 The regression equation of the standard curve is y=2.428x-21.466, R=0.9999; the injection volume has a good linear relationship in the range of 0.3μg-20μg.
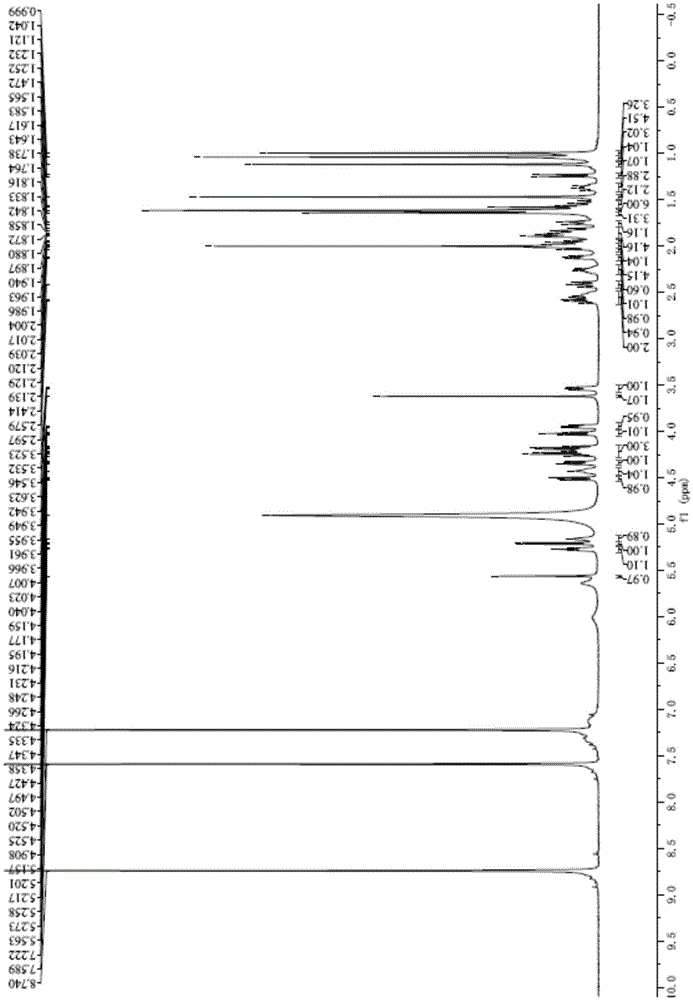
[0062] (2) Ginsenoside F 1 structural analysis of
[0063] Using high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy, the purified product was characterized, and ginsenoside F 1 MS and NMR spectral features. The instruments selected were Orbitrap Elite mass spectrometer (ThermoScientific, Bremen, Germany) and BrukerDRX-500 n...
Embodiment 3
[0075] Determination of Ginsenoside F by MTT (Tetrazolium Salt) Method 1 Killing effect on human tumor cells.
[0076] (1) Experimental design
[0077] Tumor cell lines used: A549 human non-small cell lung cancer cells, MGC80-3 human gastric cancer cells, and MCF-7 human breast cancer cells. MEM medium).
[0078] Test group:
[0079] Ginsenoside F 1 Group: 1, 25, 50, 100, 200μg / L;
[0080] Ginsenoside Rg 3 Group: 1, 25, 50, 100, 200μg / L;
[0081] Blank control group: cell culture medium;
[0082] Solvent control group: cell culture medium and DMSO;
[0083] Positive control group: 5-fluorouracil (10μg / L)
[0084] (2) method
[0085] Take the cells in the logarithmic growth phase, add 0.25% trypsin to digest, centrifuge at 600r / min for 10min, and adjust the cell concentration to 6×10 4 cells / mL, inoculated in a 96-well culture plate (edge wells were filled with sterile PBS), 90 μL per well. After culturing for 24 hours, add the sample ginsenoside F 1 (sample is d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com