Polymerizable NIR (near infrared) fluorescent dye monomer as well as preparation method and application thereof
A technology of fluorescent dyes and dye monomers, applied in the field of near-infrared fluorescent functional dye monomers, can solve the problems of low quantum yield, biological system instability, low detection sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
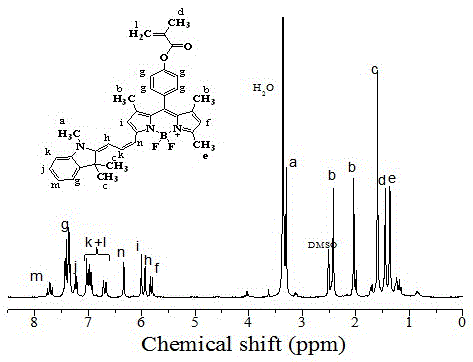
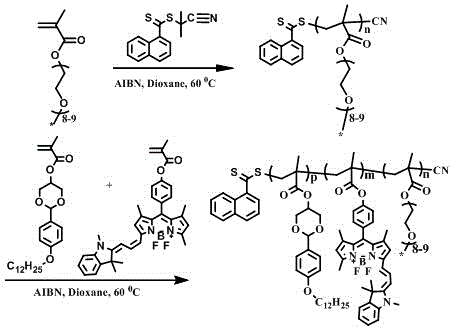
[0032] Add 105 mg of the compound fluoroborate dye and 84 μL of triethylamine into 20 mL of anhydrous tetrahydrofuran and stir to mix, and slowly add 39 μL of methacryloyl chloride diluted with 1 mL of anhydrous tetrahydrofuran dropwise in an ice-water bath. After 1 h, the ice-water bath was removed, and stirring was continued for 5 h at room temperature. Then the solvent is removed by vacuum rotary distillation, the solid is dissolved in dichloromethane and washed with saturated saline and distilled water, then dried with anhydrous sodium sulfate, the solvent is removed by distillation under reduced pressure, passed through a 200-300-mesh silica gel column, and then washed with a volume ratio of 1 :8 mixed solution of ethyl acetate and sherwood oil was eluted, and the eluate was collected and then distilled under reduced pressure to obtain the near-infrared fluorescent functional dye monomer NFM. Depend on image 3 As shown, the monomer in the solvent is tetrahydrofuran, the...
Embodiment 2
[0034] Applications of near-infrared fluorescent monomers
[0035] The macromolecular chain transfer agent PPEGMA is obtained by RAFT polymerization of poly(ethylene glycol) methacryloyl chloride (PEGMA). PEGMA (3087μL, 6.5mmol), CPDN (36mg, 0.13mmol) and AIBN (10.7mg, 6.5×10-2mmol) were added to the ampoule, and 2mL of 1,4-dioxane was added as a solvent. After deoxygenation, 60°C The reaction was stirred for 5h. Precipitate with a large amount of ice ether, centrifuge (10,000rpm, 13min) to obtain PPEGMA (Mn=20900g / mol). Then use PPEGMA (209mg, 0.010mol) as a macromolecular chain transfer agent, add the synthesized monomer NFM (6.5mg, 0.011mmol) and the hydrophobic monomer DBAM (69.1mg, 0.159mmol), then add AIBN (0.82mg, 0.005mmol) and 1mL of 1,4-dioxane ℃ stirring reaction for 11.75h, after precipitation with a large amount of n-hexane , and then centrifuged (10,000rpm, 13min) to obtain the polymer DDP, the molecular weight Mn=27700g / mol.
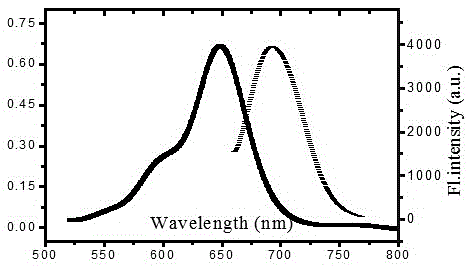
[0036] The polymer DDP (20 mg) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com