A kind of preparation method of cefditoren pivoxil intermediate
A kind of technology of cefditoren pivoxil and intermediate, which is applied in the preparation of 7-amino-3-[-2-vinyl]-3-cephem-4-carboxylic acid and the preparation of cefditoren pivoxil intermediate It can solve the problems of poor selectivity, low reaction yield and slow reaction, and achieve the effects of reduced reaction time, good selectivity and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
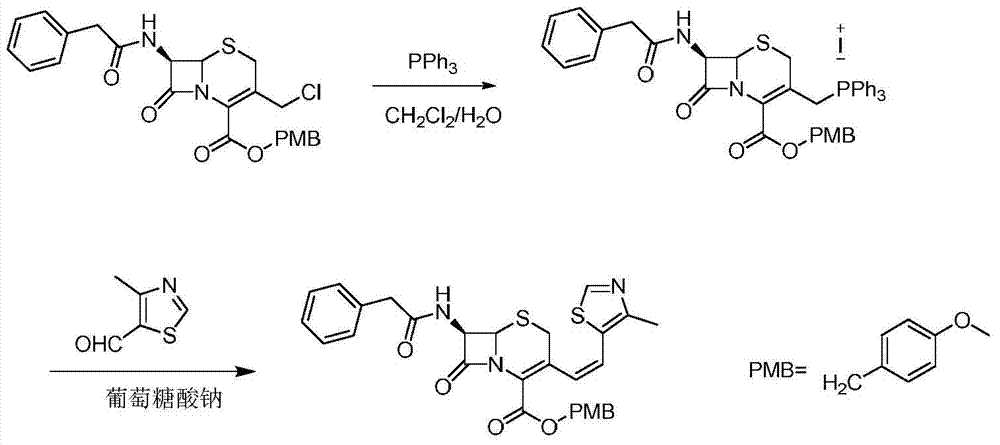
[0025] A kind of preparation method of cefditoren pivoxil intermediate, the method comprises the following steps:
[0026] 1) In the presence of sodium iodide (19.5g, 0.13mol), 7-phenylacetamido-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester (48.7g, 0.1mol) and triphenyl Phosphine (30.2g, 0.115mol) was reacted in 500mL of the first mixed solvent, then the temperature was lowered to 10°C, sodium hydroxide was added to adjust the pH to 9, stirring was continued for 15 minutes, and the layers were left to stand, and the organic layer contained The mixture of phosphorus ylides, the conditions of the contact reaction include: the contact reaction temperature is 35° C., and the reaction time is 1.5 hours, and the first mixed solvent is a mixed solvent of water and dichloromethane (the volume ratio of water and dichloromethane is 1:3);
[0027] 2) Control the temperature at 3°C, add sodium gluconate (17.9g, 0.04mol) and 4-methyl-5-thiazole formaldehyde (31.8g, 0.25mol) in...
Embodiment 2
[0029] A kind of preparation method of cefditoren pivoxil intermediate, the method comprises the following steps:
[0030] 1) In the presence of sodium iodide (16.5g, 0.11mol), 7-phenylacetamido-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester (48.7g, 0.1mol) and triphenyl Phosphine (31.5, 0.12mol) was reacted in 500mL of the first mixed solvent, then the temperature was lowered to 8°C, sodium hydroxide was added to adjust the pH to 9, stirring was continued for 20 minutes, and the organic layer contained phosphorus. The mixture of ylides, the conditions of the contact reaction include: the contact reaction temperature is 25 ° C, the reaction time is 2 hours, and the first mixed solvent is a mixed solvent of water and methylene chloride (the volume ratio of water and methylene chloride is 1 :2);
[0031]2) Control the temperature at 0°C, add sodium gluconate (35.9g, 0.08mol) and 4-methyl-5-thiazole formaldehyde (25.4g, 0.2mol) into the mixture containing phosphorus y...
Embodiment 3
[0033] A kind of preparation method of cefditoren pivoxil intermediate, the method comprises the following steps:
[0034] 1) In the presence of sodium iodide (22.5g, 0.15mol), 7-phenylacetamido-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester (48.7g, 0.1mol) and triphenyl Phosphine (28.9g, 0.11mol) was contacted and reacted in 500mL of the first mixed solvent, then the temperature was lowered to 10°C, sodium hydroxide was added to adjust the pH to 8, stirring was continued for 15 minutes, and the layers were left to stand, and the organic layer contained The mixture of phosphorus ylides, the conditions of the contact reaction include: the contact reaction temperature is 25 ° C, the reaction time is 1 hour, and the first mixed solvent is a mixed solvent of water and dichloromethane (the volume ratio of water and dichloromethane is 2:1);
[0035] 2) Control the temperature at 5°C, add sodium gluconate (26.9g, 0.06mol) and 4-methyl-5-thiazole formaldehyde (28g, 0.22mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com