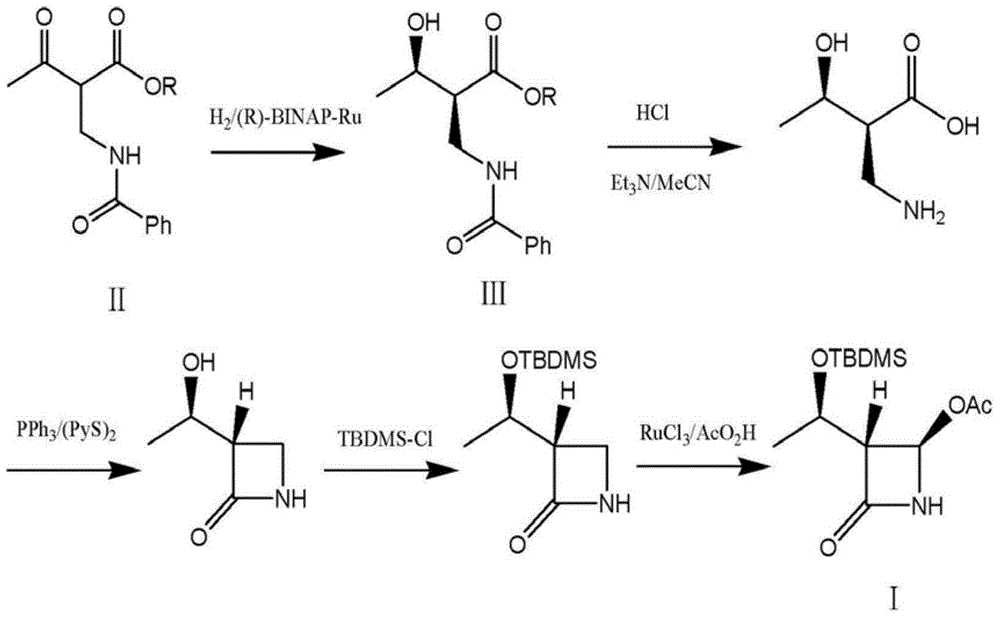
Aldo-keto reductase and application thereof in synthesis of (2S,3R)-2-benzoylaminomethyl-3-hydroxybutyrate
An aldehyde-ketone reductase and reductase technology, applied in the field of bioengineering, can solve the problems of cumbersome operation, low recovery rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 random mutation
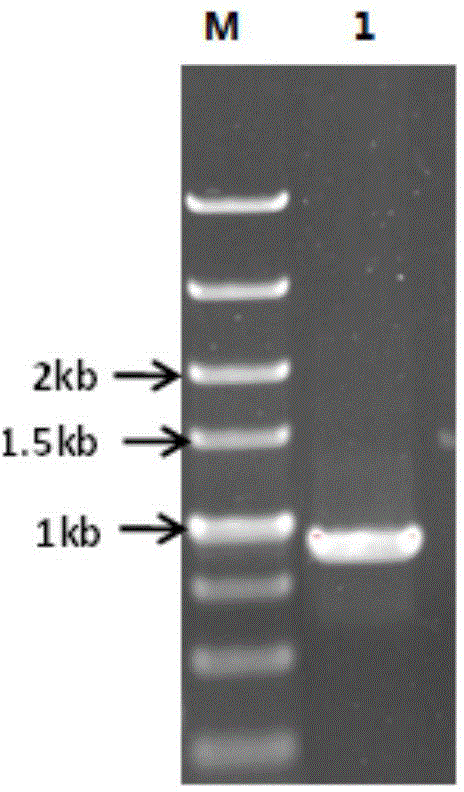
[0050] Using the aldehyde and ketone reductase gene BYKY-KRED derived from Chinese patent application 201410541899.8 (its nucleotide sequence is shown in SEQ ID NO: 1 of this application) as a template, with SEQ ID NO: 5 and SEQ ID NO: 6 as primers, BYKY-KRED was subjected to random mutation. The random mutation PCR program is as follows: 10×MutazymeII reaction buffer 5 μL, 40 mMdNTP mix 1 μL (final concentration 200 μM), forward primer and reverse primer 250 ng / μL, MutazymeII DNA polymerase 2.5 U / μL, DNA template 1 ng, ddH 2 O to make up 50 μL. PCR amplification steps are: (1) 95°C, pre-denaturation for 2min; (2) 95°C, denaturation for 30s; (3) 56°C annealing for 30s; (4) 72°C extension for 1min; steps (2) to (4) repeated 30 times; (5) Continue extending at 72°C for 10 minutes, then cool to 4°C. The PCR product was purified by agarose gel electrophoresis, and the target band in the range of 800 to 900 bp was recovered using an agarose gel...
Embodiment 2
[0051] Embodiment 2 aldehyde and ketone reductase gene isolation
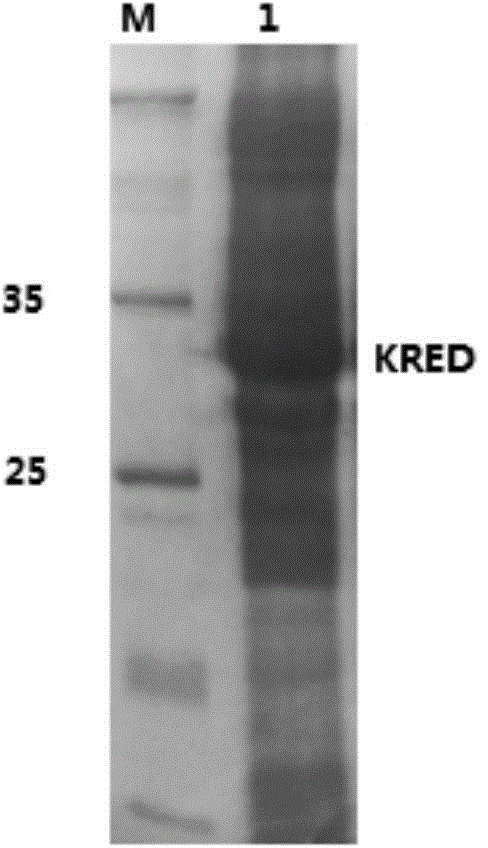
[0052]Select positive colonies and add them to a deep-well 96-well plate containing 1mL LB medium (containing 100 μg / mL ampicillin) for culture to obtain BYKY-KRED mutant genetically engineered Escherichia coli, which is inoculated into 100mL liquid LB medium for cultivation. Transfer the overnight culture to 1L of fresh LB liquid medium and grow to OD 600 When the temperature reached 0.6-0.8, IPTG was added to a final concentration of 100 μM to induce the expression of the recombinant protein, and the temperature was lowered to 30°C to continue culturing for 24 hours. Centrifuge at 5000rpm to collect the bacteria, wash once with 0.2M sodium phosphate buffer (pH7.0), and resuspend the bacteria in 500 μL of PBS lysis buffer (pH7.2) containing 1mg / mL lysozyme and 1U / mL Benzonase nuclease, Freeze and thaw 3 times at -80°C to break the bacteria, centrifuge at 10,000rpm for 10min, take 50μL of the supernatant of th...
Embodiment 3
[0053] Embodiment 3 high-density fermentation
[0054] The BYKY-KRED mutant genetically engineered Escherichia coli obtained according to Example 2 was inoculated into a 1L shake flask filled with 200 mL of LB medium, and cultured at 37° C., 180-220 rpm for 10-16 hours. The above-mentioned cultured seed culture solution was inoculated in a 3L upper tank fermentation medium (M9 medium, containing glucose 4g / L, disodium hydrogen phosphate 12.8g / L, potassium dihydrogen phosphate in a ratio of 10% (v / v). 3g / L, ammonium chloride 1g / L, sodium sulfate 0.5g / L, calcium chloride 0.0152g / L, magnesium chloride hexahydrate 0.41g / L), at 20~30℃, 300~800rpm, air flow 2~ Cultured under the condition of 6L / min. After culturing for 6-10 hours, feed medium containing 50% glycerol was fed at a rate of 5-20 mL / h until the end of fermentation. Fed feed medium for several hours to OD 600 When reaching 20-40, add 0.1-1mM IPTG to start induction. After 10 to 20 hours of induction, put the tank into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com