GSH (glutathione) sensor based on Rhodamine B and preparation and application thereof
A fluorescence sensor and reaction time technology, applied in the field of biochemistry, can solve the problems of not particularly good selectivity, low buffer solution concentration, difficult synthesis and other problems, and achieve the effects of simple synthesis steps, simple synthesis methods and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A kind of preparation method of the GSH fluorescence sensor based on Rhodamine B of the present invention comprises the following steps:
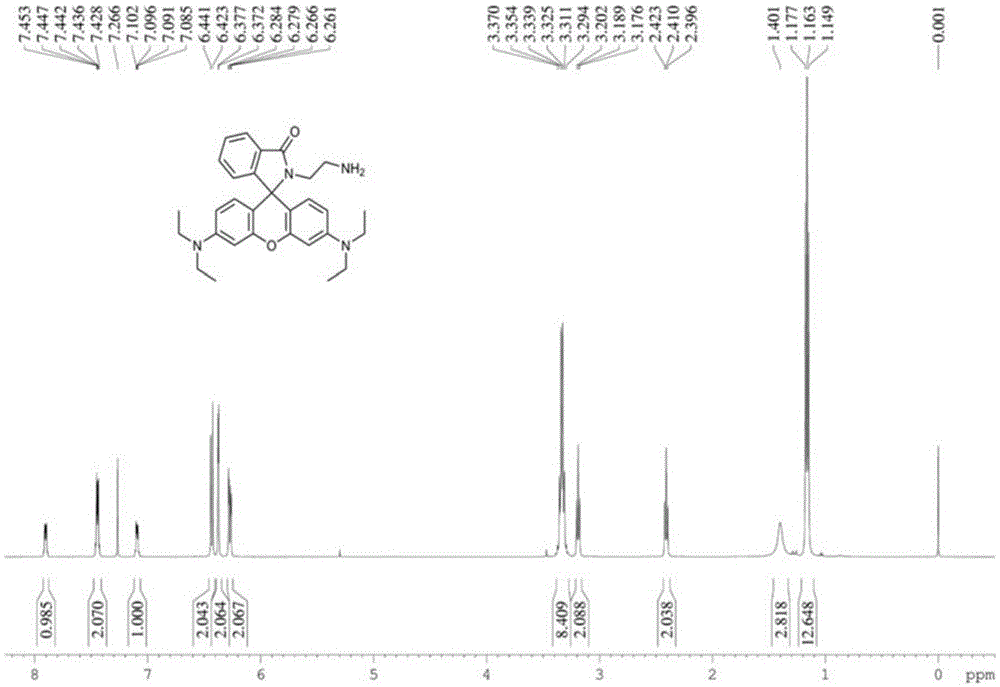
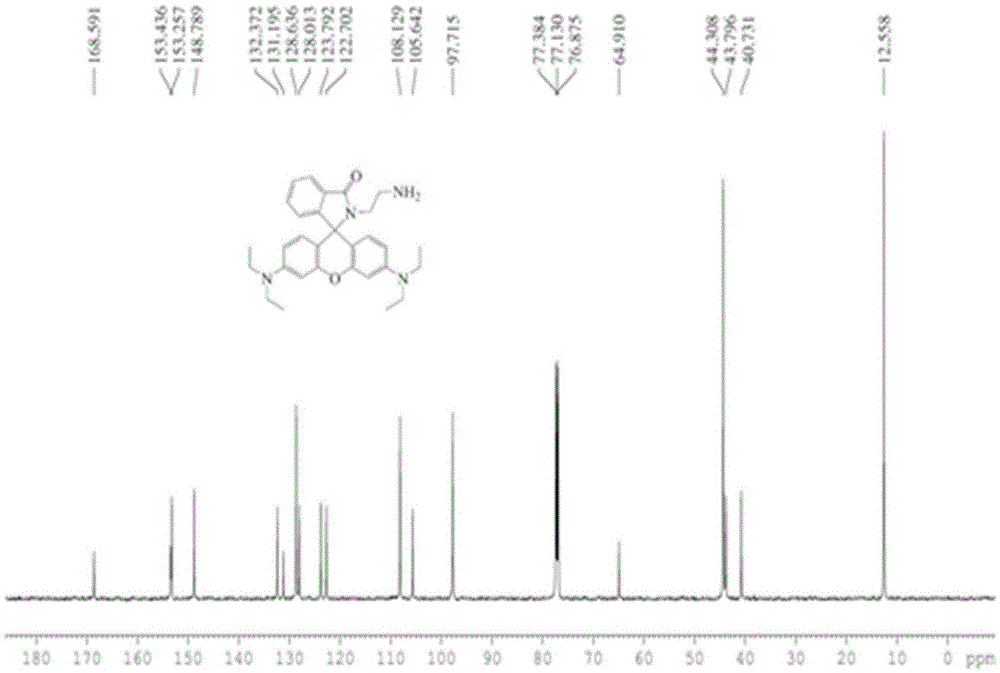
[0038] In the first step, rhodamine B and excess ethylenediamine were refluxed in absolute ethanol for more than 12 hours. After the reaction was completed, the solvent was removed under reduced pressure, extracted, and separated on a silica gel column to finally obtain a light yellow powder, that is, compound 2.
[0039] The structure of compound 2 is as follows:
[0040] ;
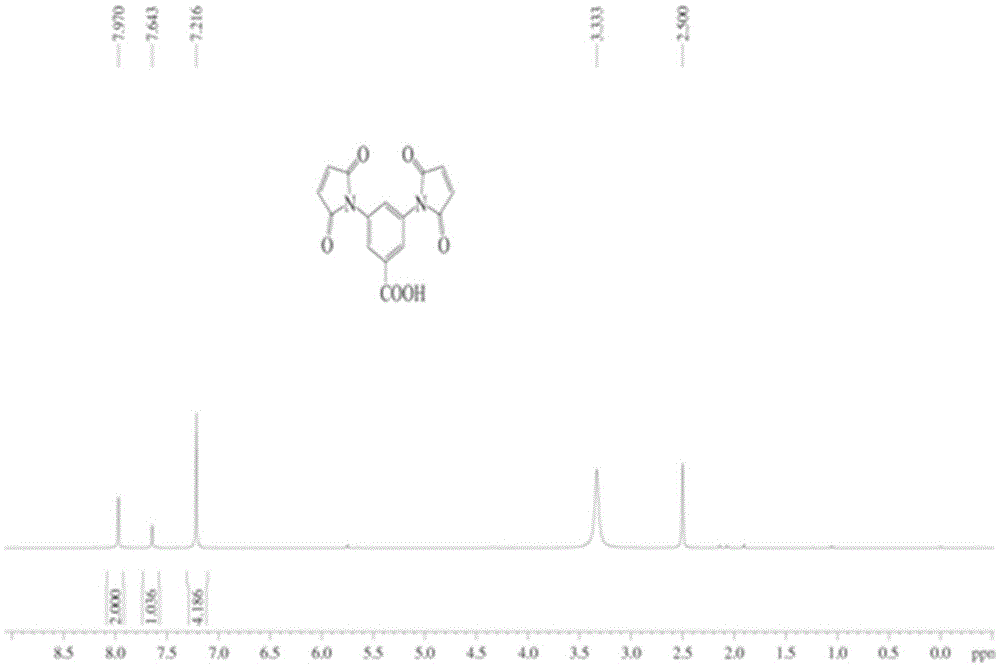
[0041] In the second step, 3,5-diaminobenzoic acid and maleic anhydride were refluxed in chloroform for 20 hours. After the reaction was completed, the solvent was removed by filtration, and the filter cake was dried to obtain a yellow-green powder, which was mixed with anhydrous sodium acetate in acetic anhydride After the reaction was completed, the reaction solution was poured into ice water and stirred for 1 hour, then the solvent was removed by filtrati...
Embodiment 1
[0048] Synthesis of embodiment 1 fluorescent chemical sensor
[0049] 1. Synthesis of compound 2
[0050] Rhodamine B (960mg, 2mmol) and ethylenediamine (1.3ml, 20mmol) were dissolved in absolute ethanol (40ml), the reaction temperature was controlled at 80°C, and the reaction time was 12h. After the reaction was completed, the solvent was removed under reduced pressure, and extracted , separated by silica gel column to obtain light yellow solid (880mg, 92%). Compound 2 1 HNMR, 13 CNMR respectively as figure 1 , figure 2 shown.
[0051] 2. Synthesis of compound 3
[0052] 3,5-Diaminobenzoic acid (200mg, 1.31mmol) and maleic anhydride (385mg, 3.93mmol) were refluxed in chloroform for 20h. After the reaction, the solvent was removed by filtration, and the solid was dried to obtain a yellow-green solid, which was reacted with anhydrous sodium acetate (35.1 mg, 0.43 mmol) in acetic anhydride for 2 h at 100 °C. After the reaction, the mixture was added to ice water and sti...
Embodiment 2
[0057] Embodiment 2 Fluorescence selection performance test
[0058] GSH fluorescent sensor 5 has good solubility in ethanol. It has been verified that compound 5 can be dissolved in EtOH:HEPES (0.6mM, pH=7.2) = 3:2 mixture, and 500ml of this solution was prepared as a stock solution (pH =7.2).
[0059] Precise configuration of GSH fluorescence sensor 5 as 1×10 -3 mol / LEtOH-H 2 O mixed solution (3 / 2, V / V), the concentration of amino acids GSH, Cys, Hcy, Lys, Ser, Gln, Gly, etc. is 5×10 -3 mol / L aqueous solution, and EtOH:HEPES (0.6mM, pH=7.2, 3 / 2, V / V) solution.
[0060] Fluorescent selectivity experiments such as Figure 6 As shown, take 3ml stock solution and place it in the liquid pool, add 120uLGSH to the solution of fluorescence sensor 5, measure its initial fluorescence intensity value, then add 120uL of various amino acid solutions prepared respectively, and measure its stable fluorescence intensity. Observed Figure 7 It can be seen that compound 5 has an obvious...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com