A kind of paeonol nano sustained-release preparation and preparation method thereof
A nano-sustained-release and paeonol technology, which is applied in pharmaceutical formulations, medical preparations of inactive ingredients, and capsule delivery, etc., can solve the problems of complex preparation conditions, production of toxic and harmful substances, complex preparation processes, etc. Simple process, increased medicinal effect and strong dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0026] A kind of paeonol nano-sustained-release preparation described in the present embodiment, its raw material ratio is:
[0027] The proportion of oil phase raw materials is: 100 milliliters of acetone aqueous solution or acetone with a mass concentration greater than 20%, 0.04 grams of paeonol, 4.0 milliliters of ethyl acetate, and 0.26 milliliters of n-butyl α-cyanoacrylate;
[0028] The raw material ratio of the water phase is: 100 ml of ultrapure water, 0.8 g of poloxamer 188, and 0.8 g of dextran-70.
[0029] A kind of preparation method of paeonol nano-sustained-release preparation described in the present embodiment, comprises the following steps:
[0030] Step 1. Accurately weigh paeonol and acetone aqueous solution or acetone, place paeonol in acetone aqueous solution or acetone and sonicate for 5 minutes, fully dissolve to obtain solution 1, and set aside;
[0031] Step 2. Accurately measure n-butyl α-cyanoacrylate and ethyl acetate and mix thoroughly, and immed...
specific Embodiment 2
[0038] A kind of paeonol nano-sustained-release preparation described in the present embodiment, its raw material ratio is:
[0039] The proportion of oil phase raw materials is: 100 ml of acetone aqueous solution or acetone with a mass concentration greater than 20%, 0.05 g of paeonol, 5.0 ml of ethyl acetate, and 0.30 ml of n-butyl α-cyanoacrylate;
[0040] The raw material ratio of the aqueous phase is: 100 ml of ultrapure water, 0.5 g of poloxamer 188, and 1.0 g of dextran-70.
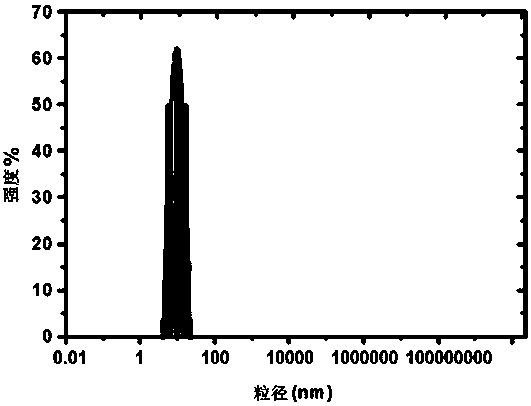
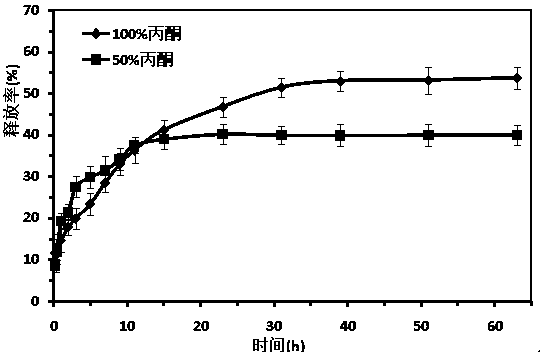
[0041] In the preparation method of a paeonol nano-sustained-release preparation described in this example, the pH value of the mixture during spontaneous interfacial polymerization is 3.0, and the remaining steps are the same as in Example 1. A kind of paeonol prepared in this example The transmission electron microscope (TEM) image, particle size visualization image and release percentage image of the skin phenol nano-sustained-release preparation are similar to those in Example 1.
specific Embodiment 3
[0042] A kind of paeonol nano-sustained-release preparation described in the present embodiment, its raw material ratio is:
[0043] The proportion of oil phase raw materials is: 100 ml of acetone aqueous solution or acetone with a mass concentration greater than 20%, 0.06 g of paeonol, 6.0 ml of ethyl acetate, and 0.35 ml of n-butyl α-cyanoacrylate;
[0044] The raw material ratio of the aqueous phase is: 100 ml of ultrapure water, 0.6 g of poloxamer 188, and 0.8 g of dextran-70.
[0045] In the preparation method of a paeonol nano-sustained-release preparation described in this example, the pH value of the mixture during spontaneous interfacial polymerization is 2.0, and the remaining steps are the same as in Example 1. A paeonol prepared in this example The transmission electron microscope (TEM) image, particle size visualization image and release percentage image of the skin phenol nano-sustained-release preparation are similar to those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com