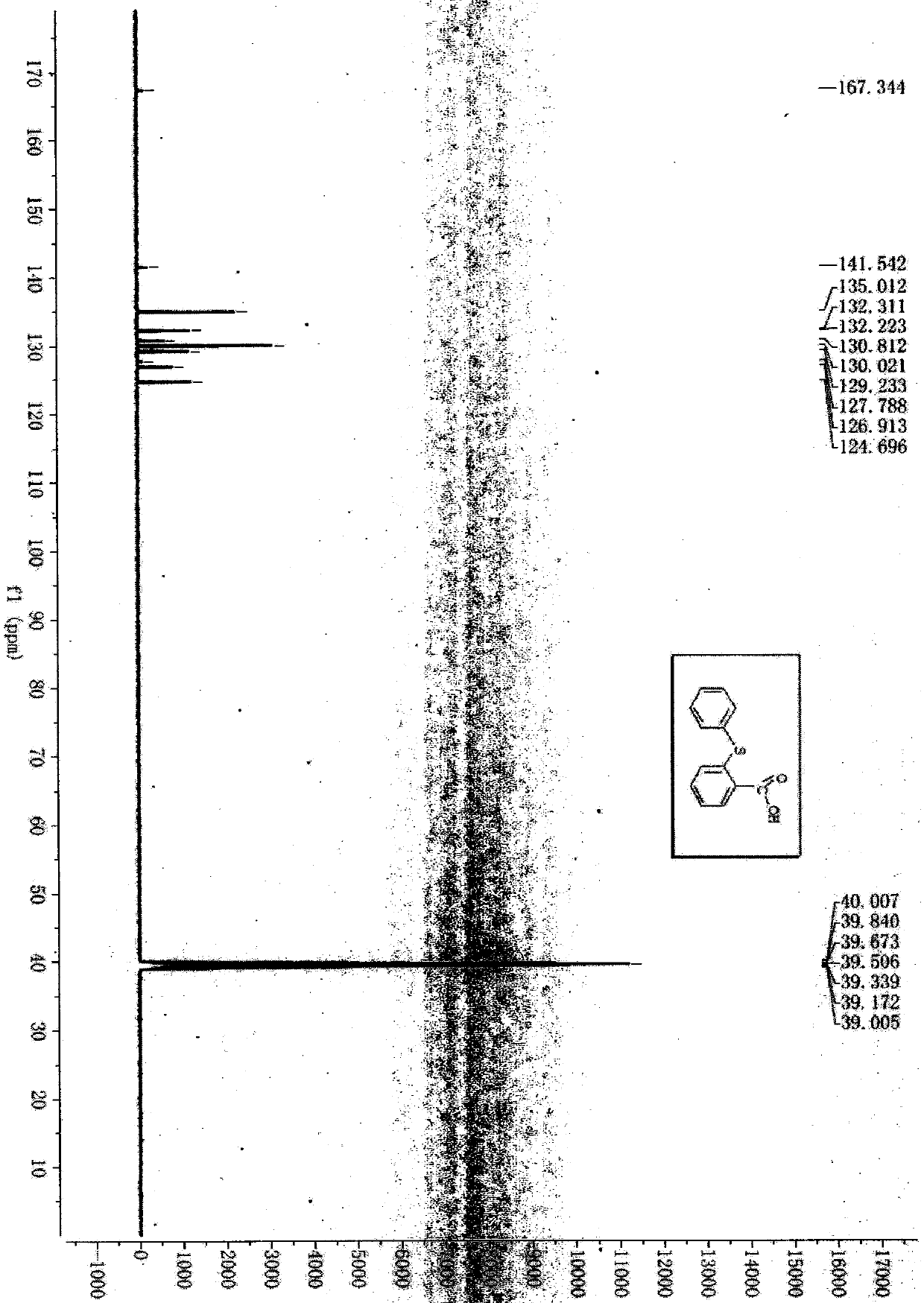
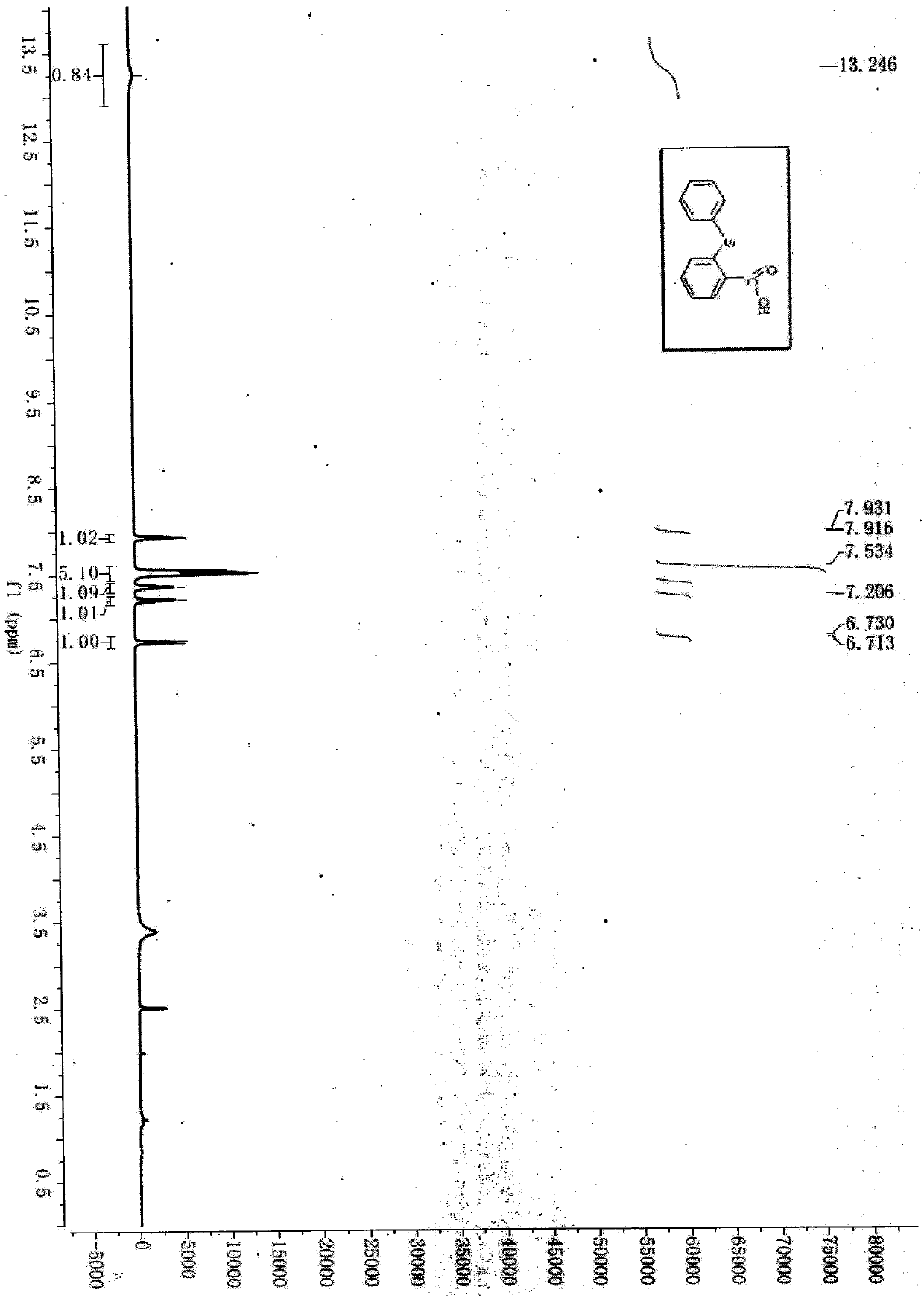
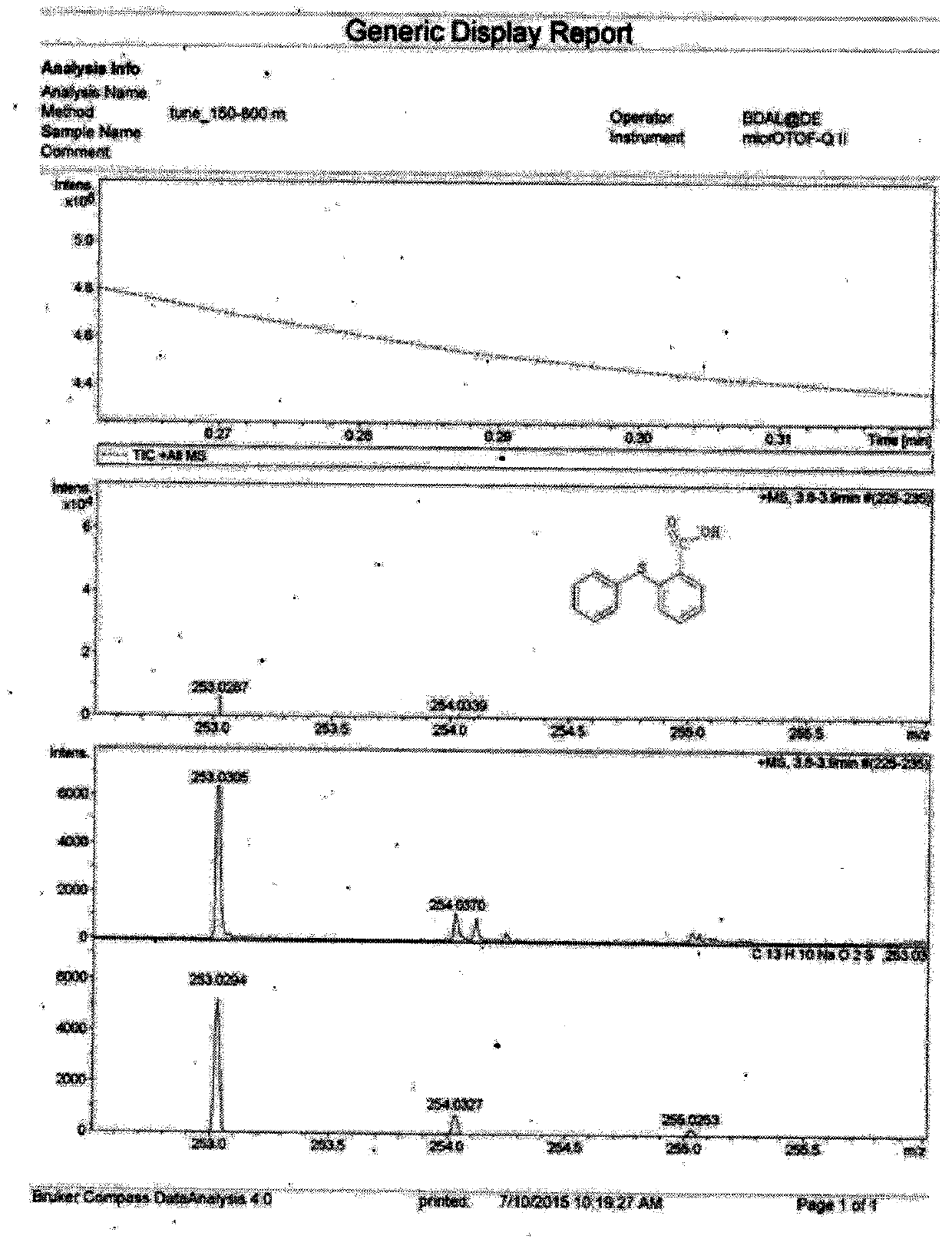
A kind of synthetic method of 2-phenylthiobenzoic acid
A kind of technology of phenylthiobenzoic acid and synthetic method, applied in the direction of thioether preparation, organic chemistry, etc., can solve the problems of expensive phosphorus ligand, expensive palladium catalyst, limit the application of palladium catalytic system, etc., and achieve easy industrial production and low toxicity Small, easy to industrialize the effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 (2: 1, sodium hydroxide, copper sulfate, DL-proline)
[0025] Add 0.35mmol (0.0539g) of thiosalicylic acid and 0.70mmol (0.0853g) of phenylboronic acid into the reaction tube, add 0.25mL of sodium hydroxide solution (10%), 0.05mL of anhydrous ethylenediamine, and then add 0.025mL of copper sulfate mmol (0.0040g) and DL-proline 0.025mmol (0.0029g) were used as catalysts, heated to 130°C for reaction, TLC tracking detection, the reaction was complete after 24 hours, TLC tracking determined that the reactants disappeared, and then naturally cooled to room temperature, the mother liquor Extracted with 170ml of ethyl acetate, took the organic layer and dried it with anhydrous magnesium sulfate, and spun to dry the solvent to obtain a solid. First pass through the column with petroleum ether with a boiling range of 60-90°C, load the crude product, then pass through the column with petroleum ether with a boiling range of 60-90°C, and then use a boiling range of 60...
Embodiment 2
[0026] Embodiment 2 (2: 1, sodium hydroxide, copper sulfate, DL-alanine)
[0027] Add 0.35mmol (0.0539g) of thiosalicylic acid and 0.70mmol (0.0853g) of phenylboronic acid into the reaction tube, add 0.25mL of sodium hydroxide solution (10%), 0.05mL of anhydrous ethylenediamine, and then add 0.025mmol of copper sulfate (0.0040g) and DL-alanine 0.025mmol (0.0022g) as a catalyst, heated to 130 ° C for reaction, TLC tracking detection, the reaction was complete in 24 hours, TLC tracking determined that the reactant thiosalicylic acid disappeared, and then cooled naturally After reaching room temperature, the mother liquor was extracted with 170 ml of ethyl acetate, the organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated to dryness to obtain a solid. First pass through the column with petroleum ether with a boiling range of 60-90°C, load the crude product, then pass through the column with petroleum ether with a boiling range of 60-90°C, and th...
Embodiment 3
[0028] Embodiment 3 (2: 1, sodium hydroxide, copper sulfate, 8-hydroxyquinoline)
[0029]Add 0.35mmol (0.0539g) of thiosalicylic acid and 0.70mmol (0.0853g) of phenylboronic acid into the reaction tube, add 0.25mL of sodium hydroxide solution (10%), 0.05mL of anhydrous ethylenediamine, and then add 0.025mmol of copper sulfate (0.0040g) and 0.025mmol (0.0036g) of 8-hydroxyquinoline as a catalyst, heated to 130 ° C for reaction, TLC tracking detection, the reaction was complete in 24 hours, TLC tracking determined that the reactant thiosalicylic acid disappeared, and then cooled naturally After reaching room temperature, the mother liquor was extracted with 170 ml of ethyl acetate, the organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated to dryness to obtain a solid. First pass through the column with petroleum ether with a boiling range of 60-90°C, load the crude product, then pass through the column with petroleum ether with a boiling range ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com