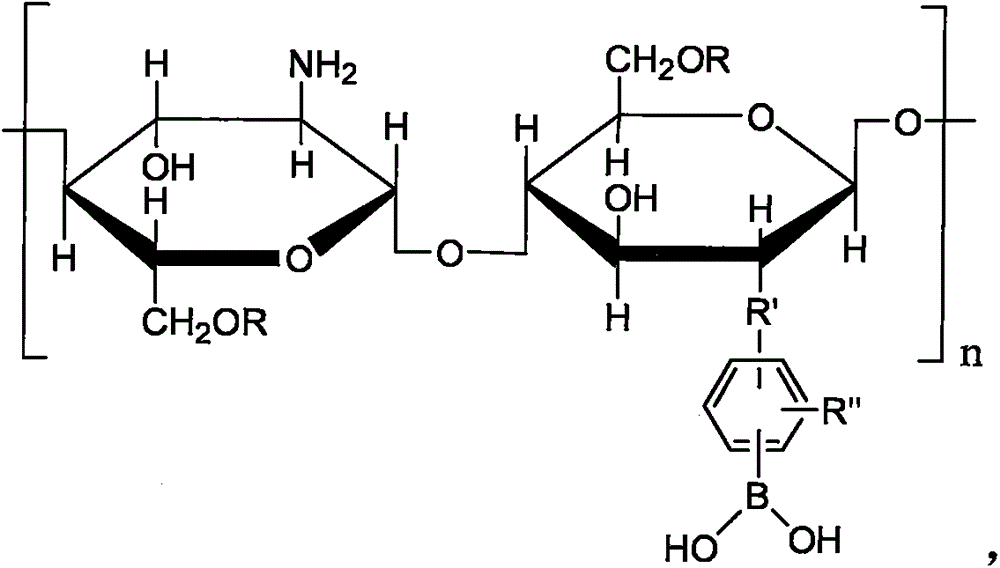
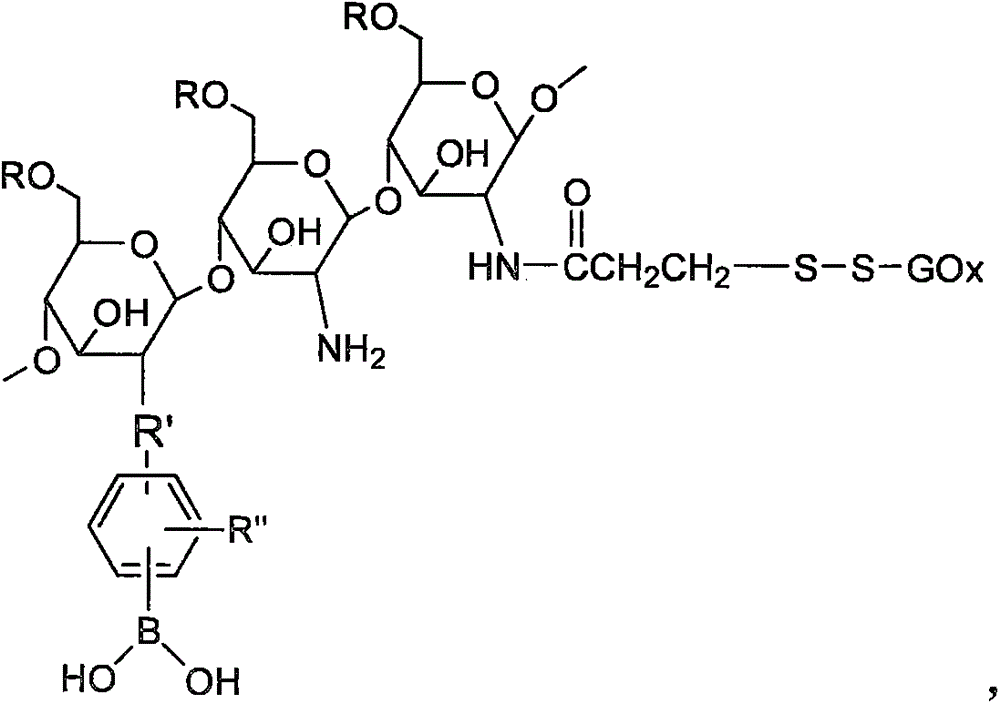
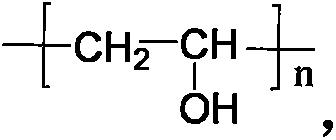
Preparation method and application for double-response bi-crosslinked injectable hydrogel used for fine-controlled release of insulin
A dual-response and dual-crosslinking technology, applied in the field of biomedicine, can solve few problems, and achieve the effects of convenient operation, synergistic therapy, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of dual-response double-crosslinked hydrogels based on phenylboronic acid / glucose oxidase modified chitosan (CSPBA-GOx) and polyvinyl alcohol / benzoyl polyethylene glycol (PVA / PEGCHO).
[0046] (1) Synthesis of CSPBA.
[0047] Add 177mg of 3-carboxyphenylboronic acid and 123.3mg of N-hydroxysuccinimide (NHS) into a 250ml round bottom flask, add 60ml of methanol to dissolve, stir at room temperature for 20min, then add 166.3mg of 1-(3-dimethylaminopropyl)-3 - Ethylcarbodiimide hydrochloride (EDC·HCl) and chitosan solution (0.5g dissolved in 60ml 0.3% HAc), after stirring at room temperature for 24h, the reactant was transferred to a dialysis bag with a molecular weight cut-off of 12kDa Dialyzed in deionized water for 48 hours, changing the water every four hours. The dialyzed sample was freeze-dried to obtain the product as a white powder. By changing the molar ratio of 3-carboxyphenylboronic acid and chitosan, CSPBA with different grafting ratios of phenylb...
Embodiment 2
[0055] Preparation of dual-response double-crosslinked hydrogels based on phenylboronic acid / glucose oxidase modified chitosan (CSPBA-GOx) and oxidized dextran (Odx).
[0056] (1) Synthesis of oxidized dextran (Odx).
[0057] Weigh 1g of dextran and dissolve it in 30ml of water, add 0.597g of sodium periodate (dissolved in 20ml of water), and react in the dark for 24 hours at room temperature, add 0.257g of glycerol, continue to stir for 15min, and transfer the reaction mixture to In a dialysis bag, dialyze in deionized water for 48 hours, changing the water every four hours. The dialyzed sample was freeze-dried to obtain the product as a white powder. By changing the molar ratio of dextran and sodium periodate, oxidized dextran with different aldehyde content can be prepared.
[0058] (2) Preparation of CSPBA-GOx / Odx dual-response double-crosslinked hydrogel.
[0059] Weigh 10mg CSPBA-GOx and dissolve in 0.5ml water, 10mgOdx dissolve in 0.5ml water, adjust the pH value to ...
Embodiment 3
[0061] Preparation of dual-response double-crosslinked hydrogels based on phenylboronic acid / glucose oxidase modified chitosan (CSPBA-GOx) and oxidized chitosan (OCS).
[0062] (1) Synthesis of oxidized chitosan (OCS).
[0063] Weigh 1g of chitosan and dissolve it in 30ml of water, add 0.597g of sodium periodate (dissolved in 20ml of water), under the protection of nitrogen, after reacting in the dark at 0°C for 24h, add 0.257g of glycerol, continue to stir for 15min, and The reaction mixture was transferred to a dialysis bag and dialyzed in deionized water for 48 h, changing the water every four hours. The dialyzed sample was freeze-dried to obtain the product as a white powder. By changing the molar ratio of chitosan and sodium periodate, oxidized chitosan with different aldehyde content can be prepared.
[0064] (2) Preparation of CSPBA-GOx / OCS dual-response double-crosslinked hydrogel.
[0065] Weigh 10mg CSPBA-GOx and dissolve in 0.5ml water, 10mgOCS dissolve in 0.5ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com