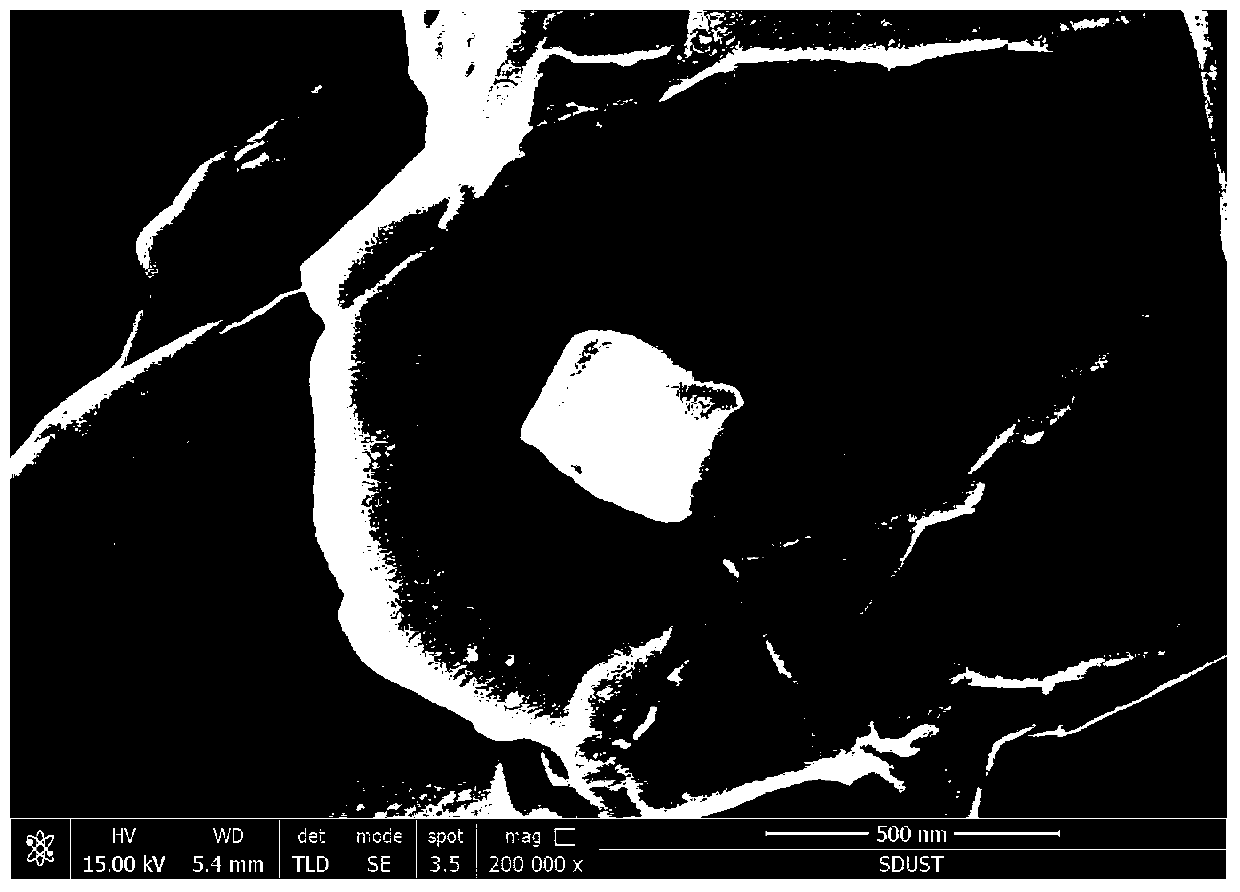
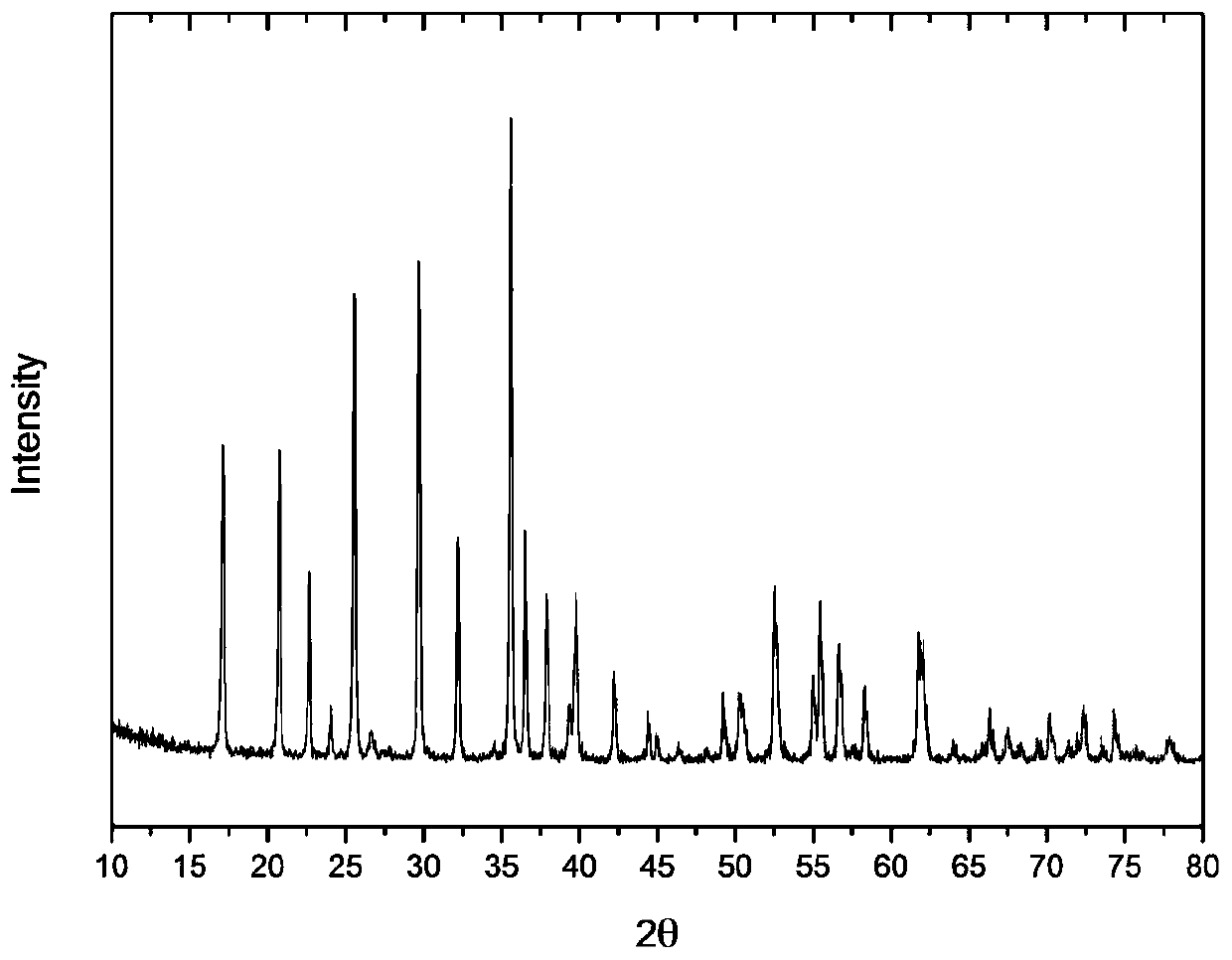
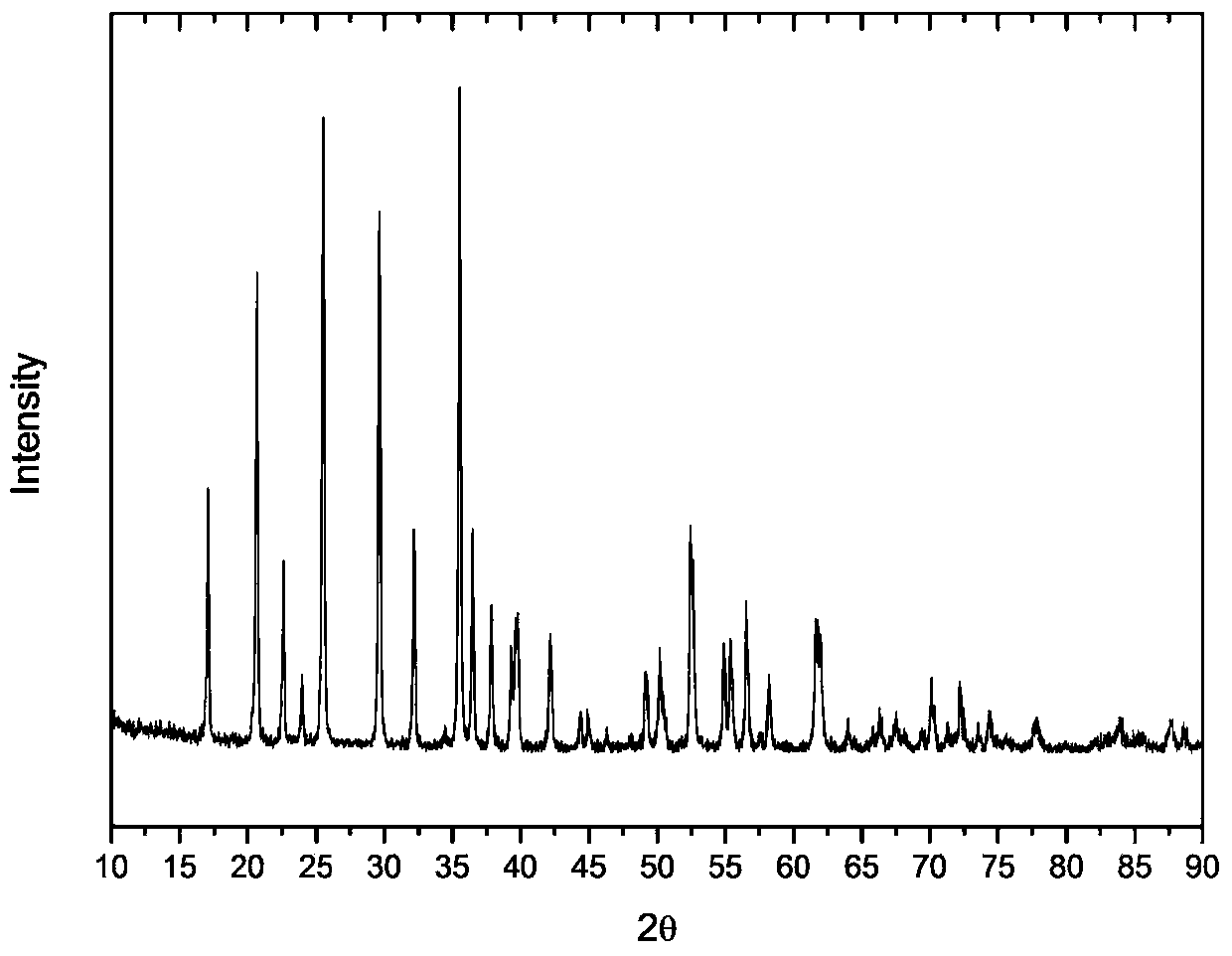
A kind of synthetic method of graphene in-situ nucleation lithium iron phosphate
A technology for nucleating lithium iron phosphate and a synthesis method, which is applied to structural parts, electrical components, battery electrodes, etc., can solve the problems of reduced rate charge and discharge capacity, large attenuation of low temperature performance of lithium iron phosphate, large interface resistance, etc. Low-temperature charge-discharge performance, improved low-temperature discharge performance, and improved electronic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) First weigh 90g Fe(OH) 2 , 3.16gVc spare;
[0032] (2) Add 1.58g of graphene oxide, 1mol of phosphoric acid and 3mol of LiOH into 1L of deoxygenated distilled water, and ultrasonically oscillate for 1 hour at room temperature to form a uniformly dispersed modified graphene oxide suspension;
[0033] (3) Fe(OH) from step (1) 2 and Vc are added to the modified graphene oxide suspension obtained in step (2), and stirred evenly at room temperature to obtain a mixed solution;
[0034] (4) Add the mixed solution obtained in step (3) into the reaction kettle, feed high-purity nitrogen gas while stirring, heat the reaction kettle to 200°C for 10 hours, cool to room temperature naturally, filter, and dry in a vacuum oven at 100°C to obtain graphite Lithium iron phosphate for in situ nucleation of alkenes.
[0035] Phosphoric acid, lithium hydroxide, and graphene oxide have active groups such as hydroxyl, carboxyl, and epoxy groups as lithium phosphate nucleation sites, an...
Embodiment 2
[0037] (1) Weigh 90g Fe(OH) 2 , 2g of hydrazine hydrate for subsequent use;
[0038] (2) Add 2g of graphene oxide, 1mol of phosphoric acid, 1mol of LiOH and 9g of polylactic acid (PLA) into 1L of deoxygenated distilled water, and ultrasonically oscillate for 1 hour to form a uniformly dispersed modified graphene oxide suspension;
[0039] (3) Fe(OH) from step (1) 2 and hydrazine hydrate are added to the modified graphene oxide suspension obtained in step (2), and stirred evenly at room temperature to obtain a mixed solution;
[0040] (4) Add the mixed solution obtained in step (3) into the reaction kettle, feed high-purity argon gas while stirring, heat the reaction kettle to 150°C for 20h, cool to room temperature naturally, filter, and dry in a vacuum oven at 60°C to obtain Lithium iron phosphate for in situ nucleation of graphene.
[0041] When PLA is added in the hydrothermal process, graphene oxide preferentially forms polylactic acid macromolecule grafted graphene wit...
Embodiment 3
[0043] (1) Weigh 90g Fe(OH) 2 , 0.45g hydrazine hydrate, 0.09g carbon nanotubes for standby;
[0044] (2) Add 4.5g of graphene oxide, 1mol of phosphoric acid and 3mol of LiOH into 1L of deoxygenated distilled water, and ultrasonically oscillate for 1 hour to form a uniformly dispersed modified graphene oxide suspension;
[0045] (3) Fe(OH) from step (1) 2 , hydrazine hydrate and carbon nanotubes are added to the modified graphene oxide suspension obtained in step (2), and stirred evenly at room temperature to obtain a mixed solution;
[0046] (4) Add the mixed solution obtained in step (3) into the reaction kettle, feed high-purity argon gas while stirring, heat the reaction kettle to 350°C for 3 hours, cool to room temperature naturally, filter, wash, and dry in a vacuum oven at 150°C , to obtain lithium iron phosphate with in-situ nucleation of graphene.
[0047] The effect of adding carbon nanotubes is that the carbon nanotubes and graphene surface nucleate lithium iron ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com