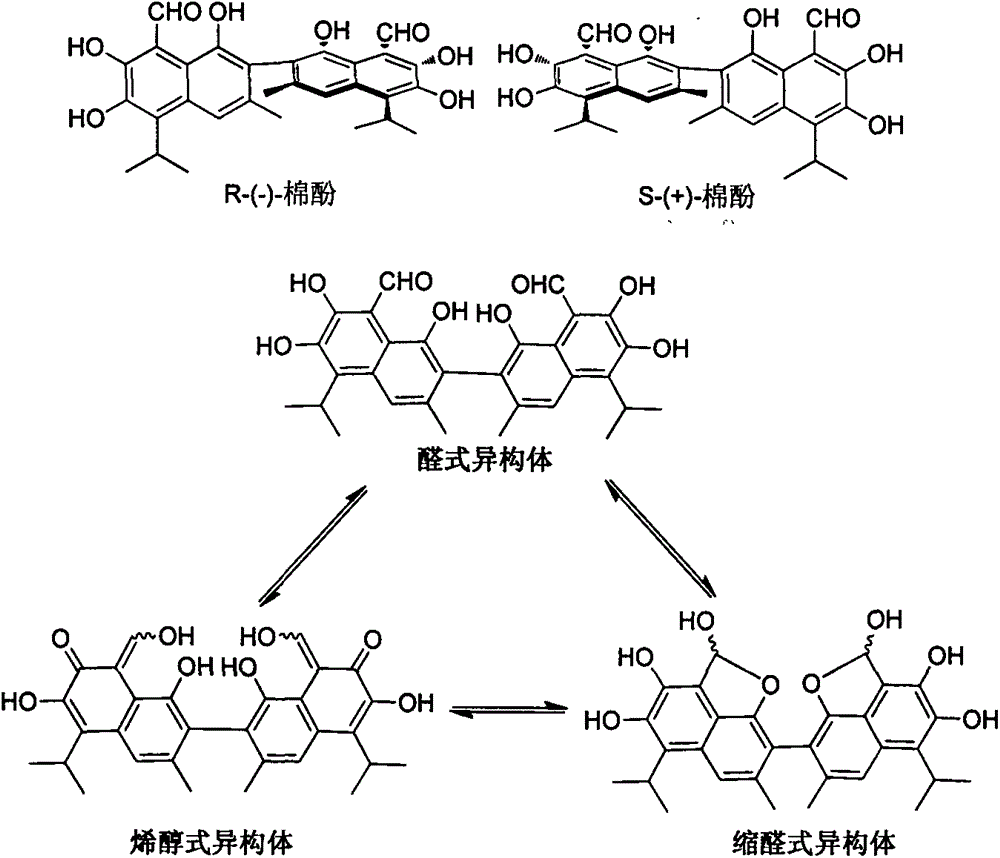
Hemigossypol derivative, vergosin derivative, preparation of hemigossypol derivative and vergosin derivative and application to pesticides
A technology of semigossypol and derivatives, which is applied in the field of pesticides and achieves the effects of good water solubility, good environmental compatibility and significant anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
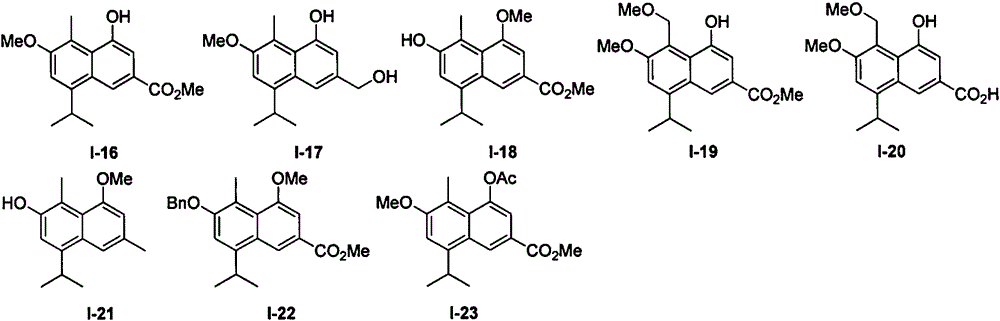
[0048] Embodiment 1: the synthesis of hemigossypol derivatives (I-1~I-4 and I-9~I-11)
[0049]
[0050]
[0051] Synthesis of 2-Hydroxy-6-isopropyl-3-methylbenzaldehyde (1)
[0052] Add 30.00g (200mmol) of carvacrol and 200mL of toluene into a 500mL four-necked bottle, respectively insert a thermometer, an air inlet pipe and a condenser tube on the four-necked bottle, and add 5.2g (20mmol) tin tetrachloride successively with a dropper under the protection of argon. ), triethylamine 8.08g (80mmol), after stirring at room temperature for 20min, add 13.19g (439mmol) of paraformaldehyde, heat and stabilize at 100°C and react for 8h, cool down, add dilute hydrochloric acid to adjust the pH value to 2, then add ethyl acetate Ester separation, the aqueous phase was extracted once with ethyl acetate, the organic phases were combined, extracted once with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered with suction, and precipitated to obtain a...
Embodiment 2
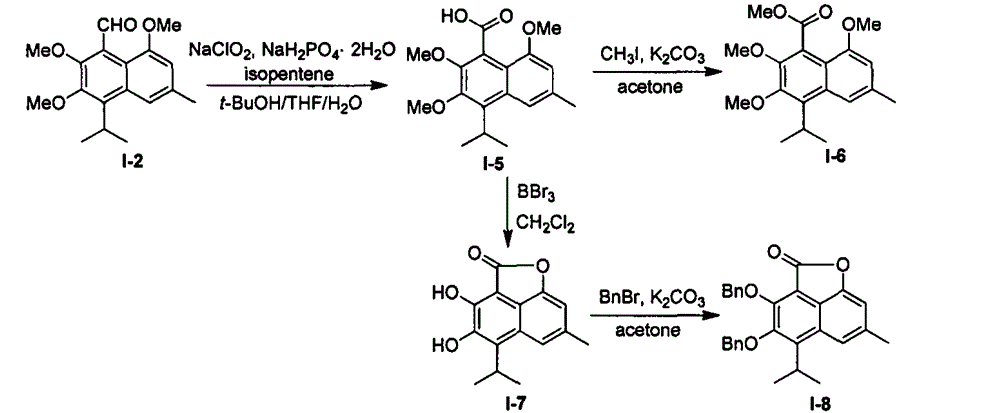
[0072] Embodiment 2: Semigossypol acid and related derivatives (I-5~I-8)
[0073]
[0074] Add 2.00g (6.61mmol) of compound I-2, 100mL of tert-butanol and 100mL of tetrahydrofuran into a 500mL round-bottomed flask. Sodium chlorite 1.50g (content > 80%, 13.27mmol) and sodium dihydrogen phosphate dihydrate 4.13g (26.46mmol) 40mL aqueous solution, slowly raised to room temperature, reacted for 10h, added saturated ammonium chloride aqueous solution 100mL, divided Liquid and aqueous phases were extracted twice with ethyl acetate, the organic phases were combined, extracted with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, suction filtered, precipitated, and recrystallized from petroleum ether and ethyl acetate to obtain compound I-5. White solid 1.68g (5.28mmol), yield: 80.0%; melting point: 190-191°C; 1 H NMR (400MHz, CDCl 3 )δ7.54(s, 1H), 6.69(s, 1H), 4.02-3.91(m, 10H), 2.50(s, 3H), 1.50(d, J=7.1Hz, 6H); 13 C NMR (100MHz, CDCl 3 )δ175.48, 154...
Embodiment 3
[0078] Example 3: Synthesis of 3,8-dimethyl-5-isopropyl-6-methoxy-1,7-naphthalenediol (I-12):
[0079]
[0080] Add 3.00g (6.87mmol) of compound I-9, 100mL tetrahydrofuran, and 0.52g (13.75mmol) of lithium aluminum hydride into a 250mL round-bottomed flask. After stirring at room temperature for 1 hour, under an ice-water bath, slowly add dilute hydrochloric acid with a dropper to No more flocs, separate the liquids, extract the aqueous phase twice with ethyl acetate, combine the organic phases, extract with saturated saline solution, dry over anhydrous magnesium sulfate, filter with suction, and desolventize to obtain the crude product. Add 120mL of methanol, 0.3mL of concentrated hydrochloric acid, 0.30g of 10% Pd / CO, pass through hydrogen to react at room temperature for 12h, filter with suction, spin the mother liquor to dryness, column chromatography (PE:EA=50:1) to obtain compound I- 12. 1.36g (5.22mmol) of light gray solid; yield: 76.0%; melting point: 147-149°C; 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com