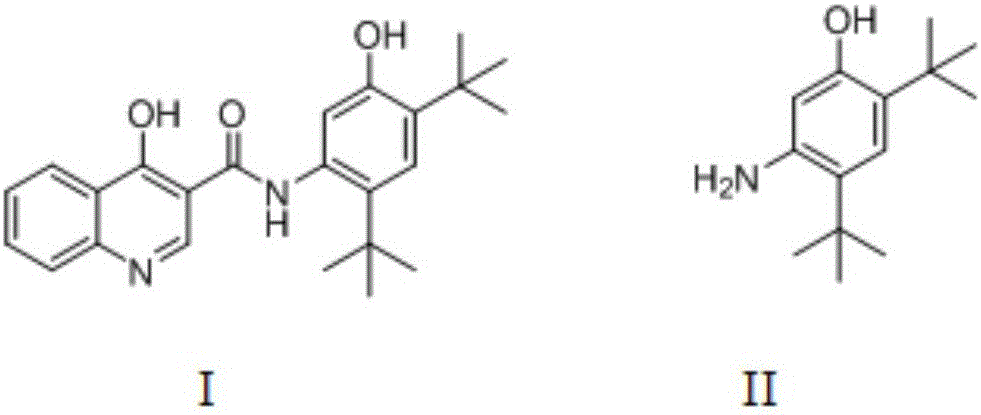
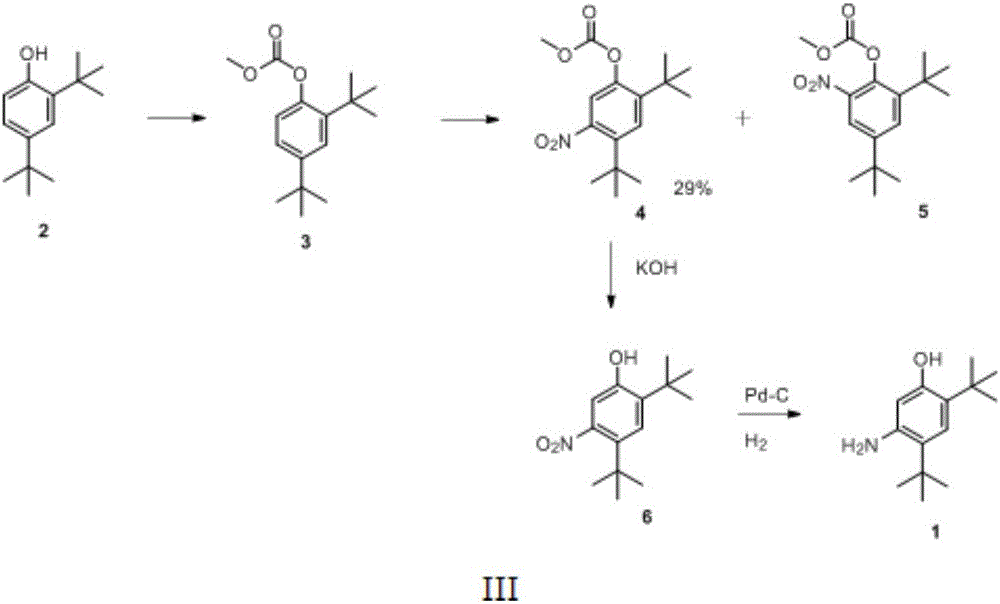
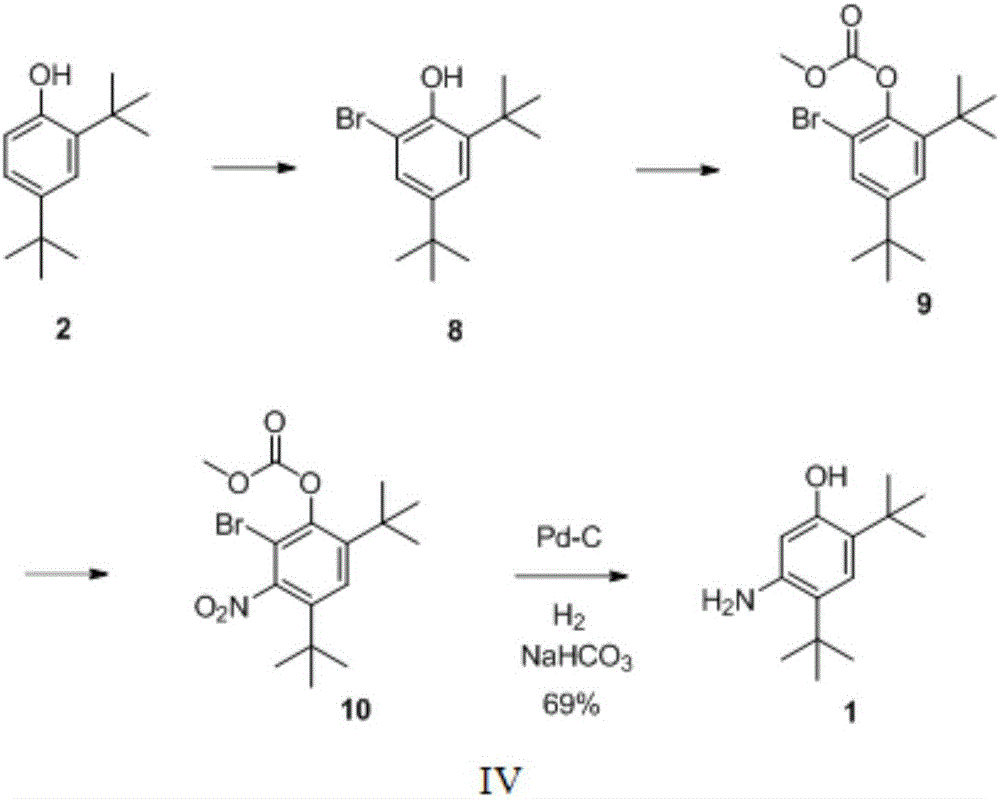
Preparation method of 2,4-ditertbutyl-5-aminophenol
A technology of di-tert-butyl and aminophenol is applied in the preparation of amino hydroxy compounds, the preparation of organic compounds, the preparation of carboxylic acid amides, etc. Good economy, good application prospects, simple reaction conditions and raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 N-(3-hydroxyphenyl) acetamide (1)
[0039] Mix 0.1mol m-aminophenol (10.9g) with 60mL acetic acid, stir at 60°C; draw 0.12mol acetic anhydride (12.2g, 11.34mL) with a needle, and add acetic anhydride dropwise within 15min while stirring at 60°C in the mixture.
[0040] After reacting for 2 hours, the reaction solution was cooled to room temperature, poured into 300 mL of water while stirring, and continued to stir after pouring, and a white solid appeared. Stir overnight (8-12 hr), filter with suction, and dry to obtain 13.8 g of white solid with a yield of 91%.
[0041] 1 H NMR(400MHz,DMSO-d6):δ2.01(s,3H),6.42(m,1H),6.92(m,1H),7.04(m,1H),7.18(m,1H),9.33(s ,1H),9.78(s,1H).ESI-MS(m / z)152.2[M+H] + .
Embodiment 2
[0042] Example 2 Preparation of N-(2,4-di-tert-butyl-5-hydroxyphenyl)acetamide (1)
[0043] Weigh N-(3-hydroxyphenyl)acetamide (12.5g, 0.083mol) prepared in Example 1 and place it in an eggplant-shaped flask, add 300mL toluene, stir and dissolve; then add tert-butanol (23.1mL, 17.9 g, 0.25mol), and 99.8wt% concentrated sulfuric acid (16.3g, 9.0mL, 0.166mmol) was added dropwise within 10min with stirring.
[0044] Stirring was continued at room temperature (10-30°C) for 12 hours, and an off-white solid was generated, which was filtered by suction. Add this solid to 200 mL of water, add saturated NaHCO 3 The pH of the solution was adjusted to 6, suction filtered, washed with water until neutral (pH=6.8-7.2), and a white solid was obtained, which was dried to obtain 17.0 g of the solid, with a yield of 78%.
[0045] 1H NMR(400MHz,DMSO-d6):δ1.25(s,9H),1.33(s,9H),1.99(s,3H),6.45(s,1H),7.11(s,1H),9.00(s ,1H),9.16(s,1H).ESI-MS(m / z)264.3[M+H] + ,527.4[2M+H] + ,549.4[2M+Na] + . ...
Embodiment 3
[0046] Example 3 Preparation of 2,4-di-tert-butyl-5-aminophenol (1)
[0047] Add 0.038mol N-(2,4-di-tert-butyl-5-hydroxyphenyl)acetamide (10.0g) into ethanol-water solution (150mL) with a volume concentration of 80%, stir, and add 9.7 mol / L hydrochloric acid 20.6mL (0.2mol), and then reflux for 12h. Part of the solvent (about 80 mL) was distilled off under reduced pressure, and the remaining reactant was poured into 100 mL of ice water and stirred to form an off-white solid, which was suction filtered and dried. The solid was mixed with 80mL of water, stirred, and 20% sodium hydroxide was added to adjust the pH to 6 to form an off-white solid, which was suction filtered and dried. The crude product was recrystallized and purified with 70 mL of ethyl acetate / petroleum ether mixture (volume ratio of ethyl acetate to petroleum ether: 1:1) to obtain 7.4 g of a white solid product with a yield of 88%.
[0048] 1 H NMR (400MHz, DMSO-d 6 ): δ1.32(s,9H),1.34(s,9H),6.79(s,1H),7.19(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com