Polymer of phenazine structure and preparation method and application thereof
A polymer, phenazine technology, used in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., to achieve the effect of increasing the degree of conjugation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 2-fluoro-1,4-alkoxyphenazine[6,7;8,9]bis(5-bromothiophene)
[0030] 4-Fluoro-3,6-dioctyloxy-phenylenediamine (0.4g, 1mmol) and 4,5-diketone-2,7-dibromo-dithienoacene (0.22g, 1mmol) were dissolved in In acetic acid (40mL), the temperature was raised to 60°C, and the reaction was incubated for 3h. Suction filtration and washing the filter cake with ethanol gave 2-fluoro-1,4-octyloxyphenazine[6,7;8,9]dithiophene (414 mg, 73%). The obtained product was dissolved in THF / DMF (5 / 10mL), and NBS (265mg, 1.5mmol) was added at 40°C. After reacting for 5h, the organic phase was washed with water and extracted with dichloromethane, dried over anhydrous magnesium sulfate to obtain 2-fluoro - 1,4-octyloxyphenazin[6,7;8,9]bis(5-bromothiophene) (360 mg, 68%).
[0031] The NMR characterization data are: 1 H NMR (CDCl 3 ,500MHz,ppm), δ=7.23(s,2H),6.79(d,1H),3.77-4.02(m,4H),1.96(m,2H),1.29-1.33(m,16H),0.94(t ,12H).
[0032]
Embodiment 2
[0034] Preparation of Polymers Shown in Formula II
[0035]
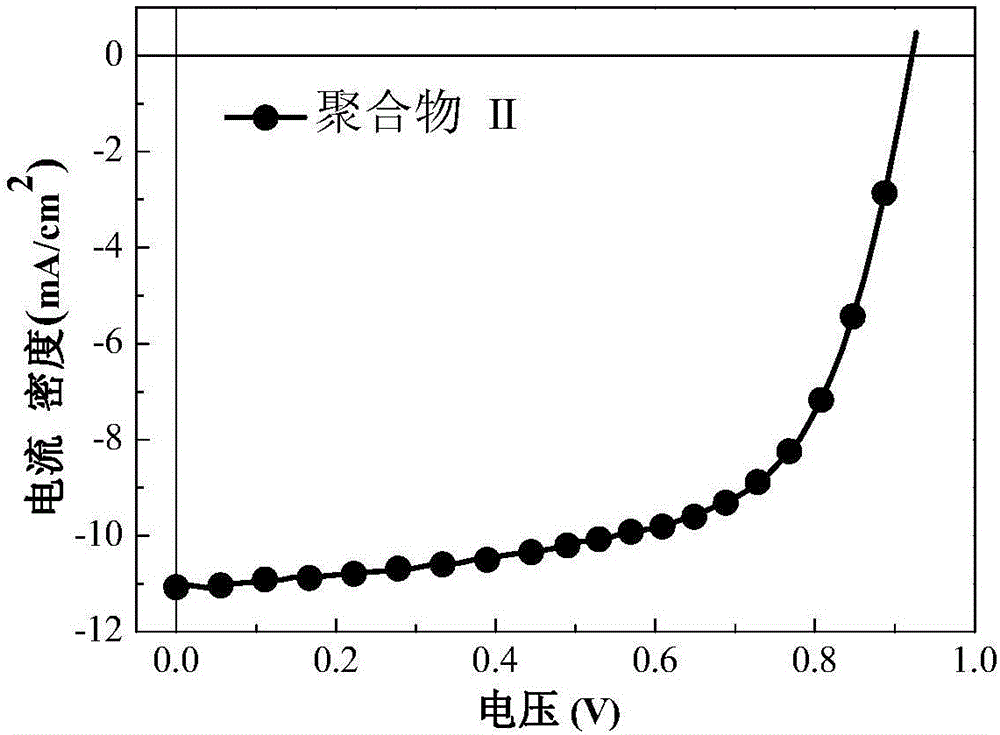
[0036] The reaction was carried out under nitrogen protection, 2-fluoro-1,4-octyloxyphenazin[6,7;8,9]bis(5-bromothiophene) (222mg, 0.3mmol) and 2,5-bis( Trimethyltin)thiophene (198.6mg, 0.3mmol) was dissolved in 10mL of toluene, nitrogen gas was passed for 0.5h, and the catalyst tris(dibenzylideneacetone)dipalladium (5.5mg, 0.006mmol) and the ligand tri-o-toluene were added Base phosphorus (9.8mg, 0.0024mmol), continue to ventilate for 0.5h, then start heating, reflux at 100°C for 24h, cool the system naturally at room temperature, add dropwise to methanol to settle, filter, collect the filter residue and bake in a vacuum oven at 50°C for 12h , the obtained polymer was sequentially extracted with methanol, n-hexane, and chloroform by Soxhlet, the chloroform extract was concentrated, dropped into methanol for sedimentation, and filtered to obtain 139 mg of polymer II with a yield of 72%, a number average molecular...
Embodiment 3
[0042] The preparation of polymkeric substance shown in formula Ⅲ
[0043]
[0044] Same as Example 2, the electron-deficient unit is 2,3-difluoro-1,4-octyloxyphenazine[6,7;8,9]bis(5-bromothiophene), obtained by the same polymerization method Polymer Ⅲ, yield 66%, number average molecular weight 36.67kDa, distribution coefficient 2.75.
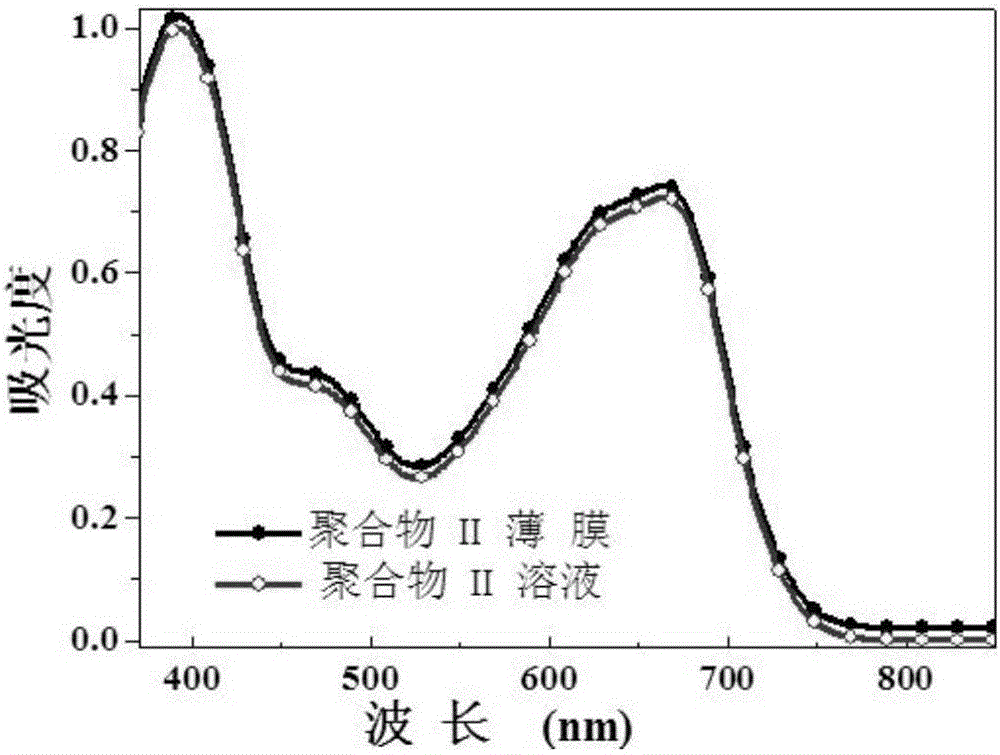
[0045] Absorption spectrum test: Polymer III was completely dissolved in chloroform and spin-coated with a homogenizer to form a film, and the solution and film were tested by UnicoUV-2102 ultraviolet-visible absorption spectrometer respectively. The optical energy gap (E g ) is 1.67eV.
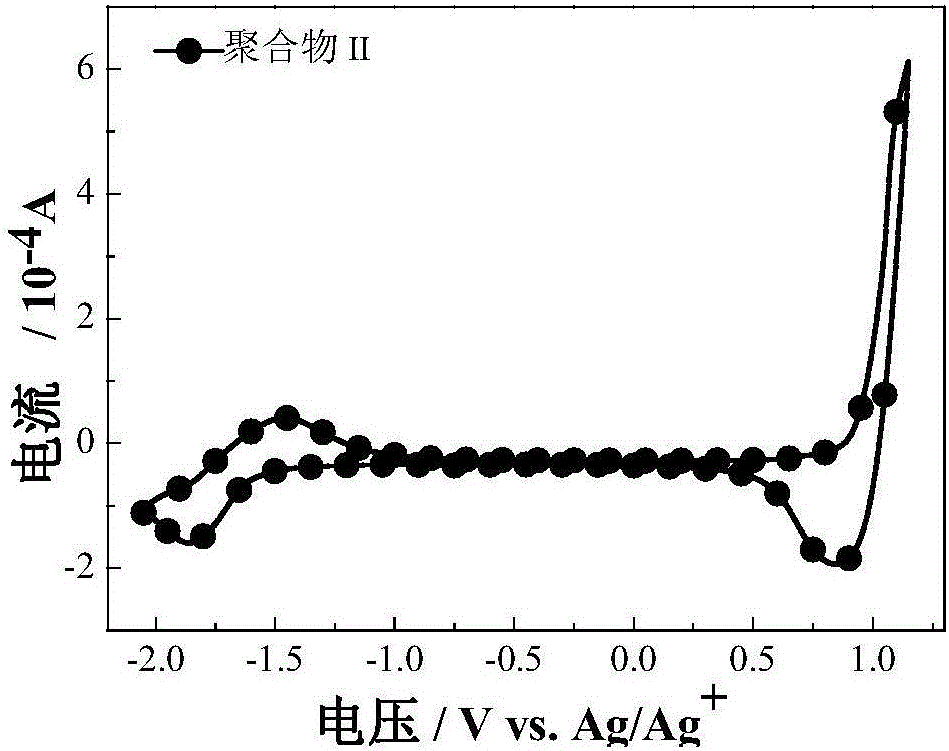
[0046] Electrochemical test: use CHI660D electrochemical workstation, use glassy carbon electrode as working electrode, platinum wire electrode as counter electrode, Ag / Ag + The electrode is the reference electrode, Bu 4 N·PF 6 As an electrolyte, in acetonitrile solvent, the HOMO energy of the polymer Ⅲ thin film was determined by cyclic voltammetry to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com