Preparation method for cubic-phase lithium lanthanum zirconium oxide solid-state electrolyte nano material
A solid electrolyte, lithium lanthanum zirconium oxide technology, applied in the direction of electrolyte immobilization/gelation, circuits, electrical components, etc., can solve the problems of low cost, difficult production, etc., to achieve improved sinterability, low price, and improved boundary conductance rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
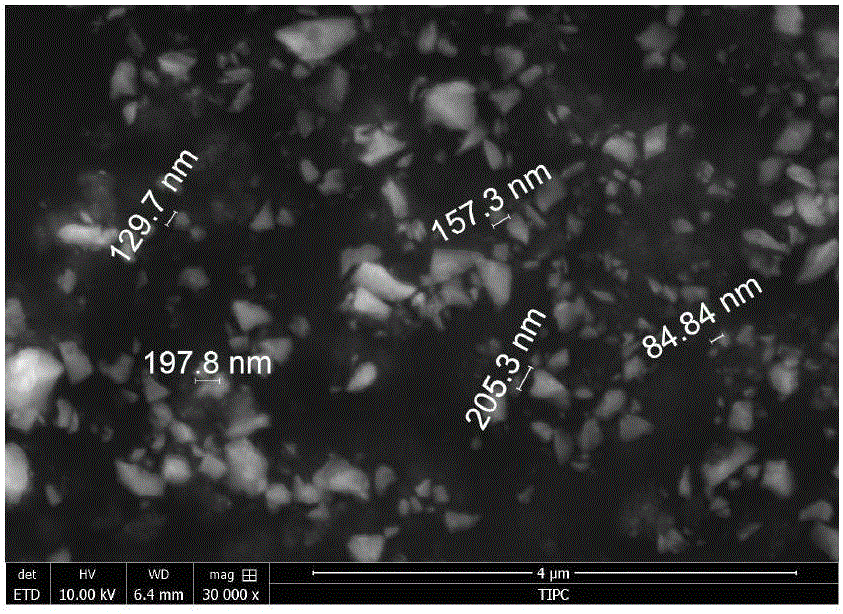
[0031] Example 1 uses nitrate and glycine as raw materials without any pretreatment. First weigh 15.4mmol of lithium nitrate, 6mmol of lanthanum nitrate, 4mmol of zirconium nitrate, 0.48mmol of aluminum nitrate and 12.94mmol of glycine, dissolve them in deionized water, put them on an electric furnace, and heat until the combustion synthesis reaction occurs after evaporation to obtain white fluffy Powder material. The powder material was cold-pressed at 300MPa into a disc with a diameter of 15mm, and then heated in a muffle furnace at 900°C for 4h to obtain a cubic phase lithium lanthanum zirconium oxide. The obtained cubic phase lithium lanthanum zirconium oxide was ball milled for 10 hours at a ball milling speed of 300r / min. The ball milled powder was again cold-pressed at 300MPa into a disc with a diameter of 15mm, and fired at 1200°C for 5 hours to form a ceramic sheet at a heating rate of 1 °C / min.
[0032] Spray gold on the upper and lower surfaces of the obtained lit...
example 2
[0033] Example 2 uses nitrate and urea as raw materials without any pretreatment. First weigh 15.4mmol of lithium nitrate, 6mmol of lanthanum nitrate, 4mmol of zirconium nitrate, 0.48mmol of aluminum nitrate and 15mmol of urea, dissolve them in deionized water, place them on an electric furnace, and heat until the combustion synthesis reaction occurs after evaporation to obtain white fluffy powder body material. The powder material was cold-pressed at 200MPa into a disc with a diameter of 15mm, and then heated in a muffle furnace at 700°C for 4h to obtain a cubic phase lithium lanthanum zirconium oxide. The obtained cubic phase lithium lanthanum zirconium oxide was ball milled for 15 hours at a ball milling speed of 300r / min. The ball milled powder was again cold pressed at 200MPa into a disc with a diameter of 15mm, and then fired into a ceramic sheet after heat treatment at 1200°C for 4 hours. The heating rate was 1 °C / min.
[0034] Spray gold on the upper and lower surfac...
example 3
[0035] Example 3 uses nitrate and glycine as raw materials without any pretreatment. First weigh 15.4mmol of lithium nitrate, 6mmol of lanthanum nitrate, 4mmol of zirconium oxynitrate, 0.48mmol of aluminum nitrate and 10mmol of glycine and dissolve them in deionized water. Powder material. The powder material was cold-pressed at 200MPa into a disc with a diameter of 10mm, and then heated in a muffle furnace at 800°C for 4h to obtain a cubic phase lithium lanthanum zirconium oxide. The obtained cubic phase lithium lanthanum zirconium oxide was ball milled for 10 hours at a ball milling speed of 300r / min. The ball milled powder was again cold pressed at 200MPa into a disc with a diameter of 15mm, and then fired into a ceramic piece after heat treatment at 1200°C for 5 hours at a heating rate of 1 °C / min.
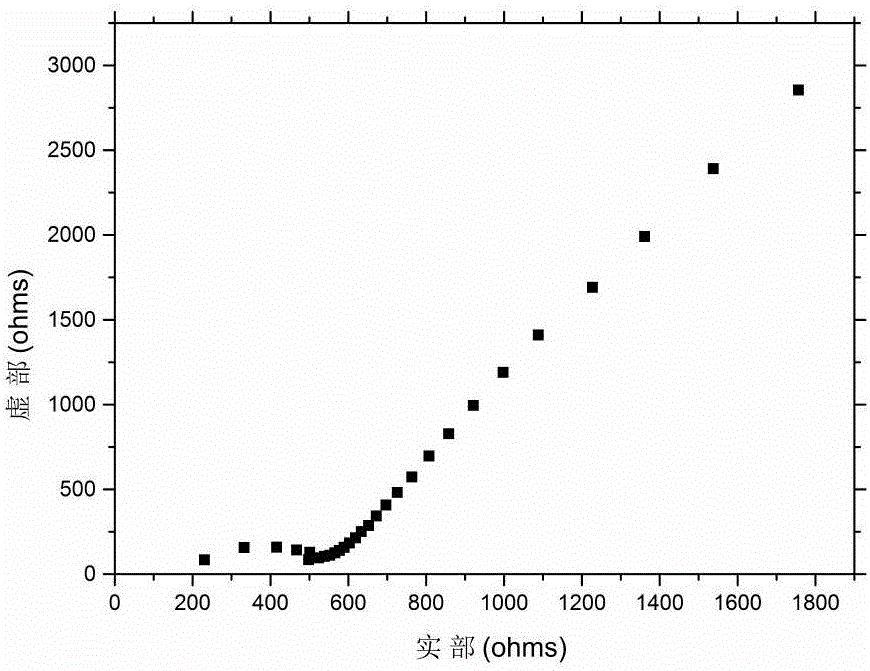
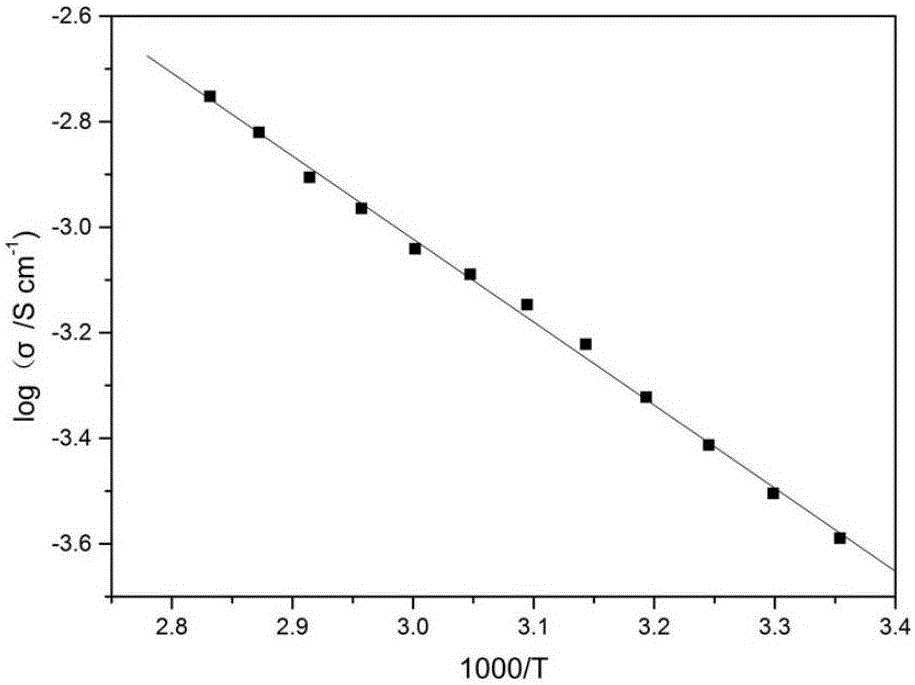
[0036] Spray gold on the upper and lower surfaces of the obtained lithium lanthanum zirconium oxide ceramic sheet, test the AC impedance at room temperature on the electrochem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com