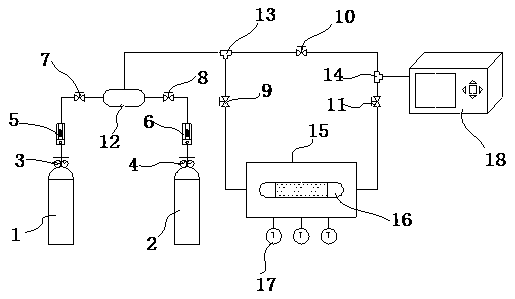
Adsorbent for separating NO in smoke and application thereof
An adsorbent and flue gas technology, applied in the field of flue gas purification, can solve the problems of adsorption capacity, selectivity, stability, energy consumption and economy. It can solve the problems of complex clusters, etc., and achieve the effects of good thermal stability and regeneration performance, easy industrial production, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1, the adsorbent of this example is made by the following method, the amount of other substances is calculated according to the amount of 1 mmol of formic acid used, 1 mmol of formic acid and 12 mmol of acrylamide are placed in the reactor, and 10 ml of acetonitrile is added, Sonicate for 12 minutes, and let the formic acid and acrylamide fully act for 1 hour; then add 25mmol of trimethylolpropane trimethacrylic ester and 0.35mmol of azobisisobutyronitrile, and ultrasonically degas for 10 minutes , filled with nitrogen at a flow rate of 100ml / min for 15 minutes, and sealed the reactor after the nitrogen replaced all the air. The reactor was placed at room temperature under UV light to initiate polymerization for 24 hours. The polymerization reaction product was ground and passed through a 100-mesh sieve, then placed in a Soxhlet extractor, and washed repeatedly for 24 hours with 150ml of a mixture of methanol with a volume ratio of 5:1 and 20% hydrochloric acid ...
Embodiment 2
[0022] Example 2, the adsorbent of this example is made by the following method, the consumption of other substances is calculated according to the consumption of 1 mmol of acetic acid, 1 mmol of acetic acid and 8 mmol of acrylamide are placed in the reactor, and 16 ml of acetonitrile, After 4ml of toluene, sonicate for 15 minutes, and let it stand for 1.2 hours to make the acetic acid and acrylamide fully act; then add 18mmol of divinylbenzene and 0.35mmol of azobisisobutyronitrile, and after ultrasonic degassing for 8 minutes, The flow rate of 120ml / min was filled with nitrogen for 13 minutes, and the nitrogen replaced all the air to seal the reactor. The reactor was placed in a constant temperature water bath at 55°C, and the polymerization reaction was initiated for 36 hours. The polymerization reaction product was ground and placed in a Soxhlet extractor after passing through a 100 mesh sieve, and washed repeatedly for 30 hours with a mixture of 8:1 methanol and 20% hydro...
Embodiment 3
[0023]Example 3, the adsorbent of this example is made by the following method, and the dosage of other substances is calculated according to the dosage of 1 mmol of oxalic acid, 1 mmol of oxalic acid and 10 mmol of acrylamide are placed in the reactor, and After 15ml of acetonitrile, sonicate for 11 minutes, and let stand for 1 hour to make oxalic acid and acrylamide fully act; then add 20mmol of divinylbenzene and 0.4mmol of azobisisoheptanonitrile, and ultrasonically degas for 8 minutes Afterwards, nitrogen gas was charged at a flow rate of 140 ml / min for 11 minutes, and the reactor was sealed after the nitrogen gas completely replaced the air. The reactor was placed in a constant temperature water bath at 60°C, and the polymerization reaction was initiated for 48 hours. The product is ground and placed in a Soxhlet extractor after being crossed through a 100 mesh sieve, and washed repeatedly for 28 hours with a mixture of methanol and 20% hydrochloric acid at a volume rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com