{0><}0{>Radio-labeled tumor developing agent as well as preparation method and application thereof
A tumor imaging agent and labeling technology, applied in the field of medical imaging, can solve problems such as weak amino acid targeting, and achieve the effects of good biological evaluation effect, efficient response, and short labeling time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the synthesis of labeling precursor namely compound 7-1 and compound 7-2
[0050] 1) In 10ml of tetrahydrofuran, add compound 1 (0.99g, 0.01mol), compound 2 (1.45g, 0.012mol), add tetraethoxytitanium (4.56g, 0.02mol), stir and react at room temperature overnight; add 10ml After diluting with ethyl acetate, add 10ml of saturated sodium chloride solution to quench, stir for 5min, filter through celite to remove insoluble matter, take the organic phase of the filtrate, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, remove the solvent, The crude product was eluted with a dichloromethane-ethyl acetate mixture with a volume ratio of 10-20:1 and purified by silica gel column chromatography to obtain compound 3, 1.63 g;
[0051] 2) In the glove box, compound 3 (0.2 g, 1 mmol) was added to 2 ml of toluene; another compound 4 (0.51 g, 2 mmol) was dissolved in 2 mL of toluene, and added to the solution containing compound 3; then (1 ...
Embodiment 2
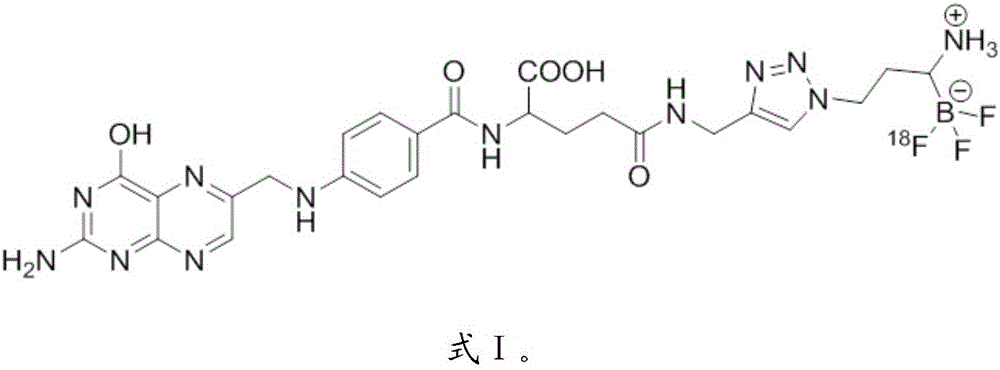
[0054] Example 2: 18 Preparation of F-labeled tumor imaging agent, compound 9
[0055] (1) Preparation of compound 7-2
[0056] a) will 18 F is eluted from the anion trapping column QMA with a mixed solution of potassium carbonate and Kriptofix 2.2.2 (K2.2.2) (mass ratio 5:1) to obtain a K of 5-20 mCi 18 F aqueous solution;
[0057] b) Add 7.5 μL of pyridazine-hydrochloride buffer solution with a pH of 2.5, 7.5 μL of N,N-dimethylformamide, and compound 7-1 prepared in Example 1, 1 mg, into a 1.5 mL centrifuge tube;
[0058] c) the K obtained in step a) 18 F aqueous solution (5~20mCi, 10μL) was added to the solution in step b), heated to 60°C for 10min, cooled, and then diluted with 2ml of deionized water, followed by C18Sep-Pak cartridge column chromatography, first deionized with 2ml Deionized water was used to remove impurities, and then the product was rinsed with a mixture of 0.5ml boric acid and 1ml of PBS-ethanol solution with a volume ratio of 1:1 as the eluent to ...
Embodiment 3
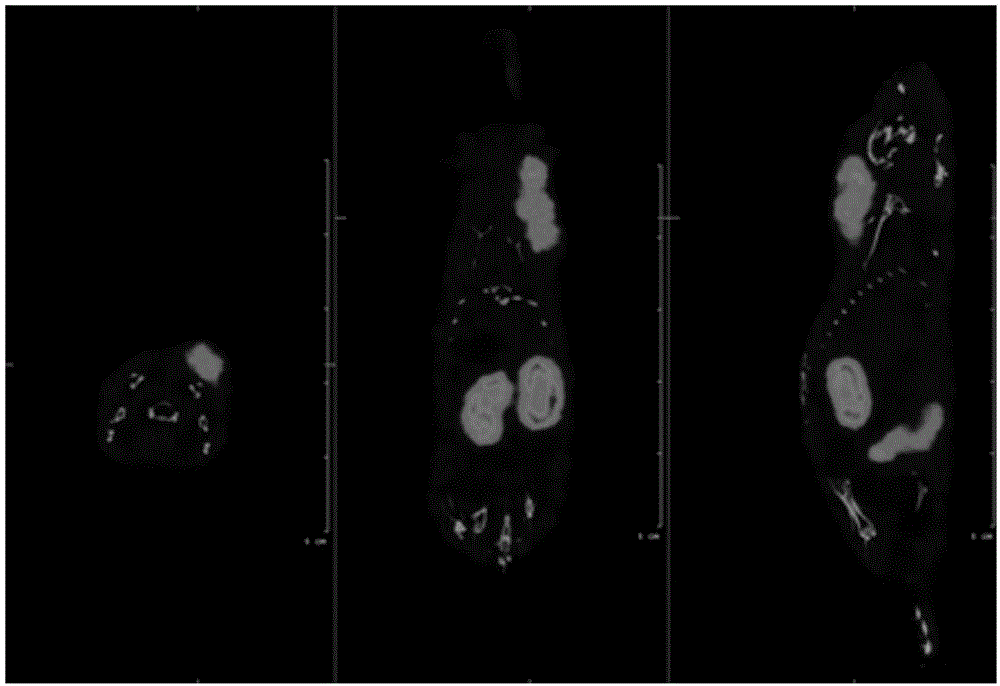
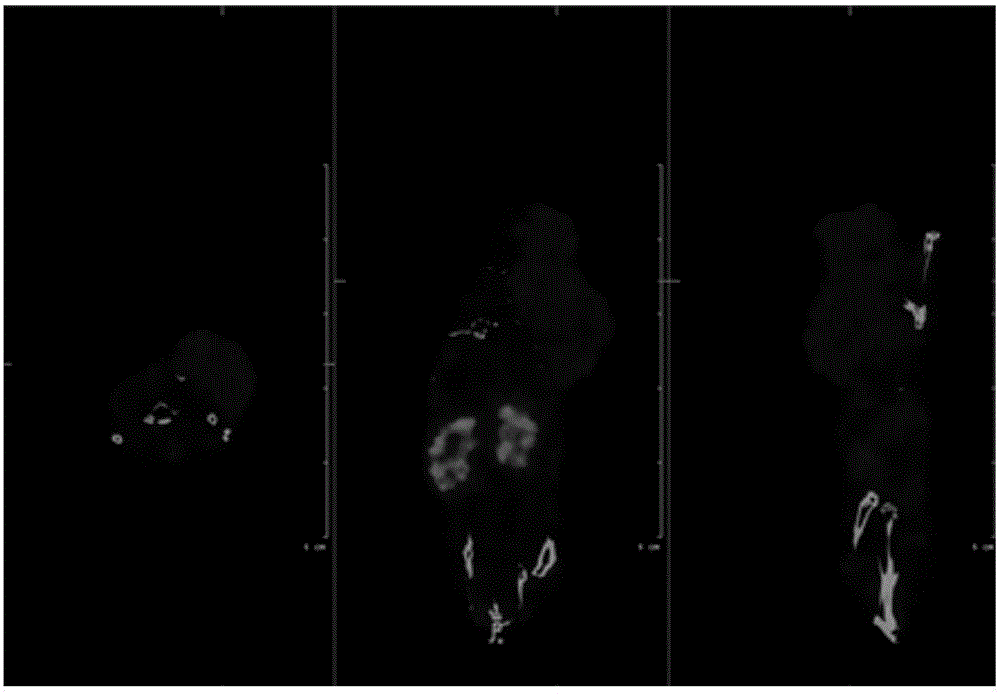
[0060] Example 3: 18 Biodistribution of F-labeled tumor imaging agent (compound 9)
[0061] Select 18-20g nude mice, and inject about 5×10 cells into the axilla of the forelimb of the nude mice 6 KB cell fluid modeling. During the first week of the experiment, folic acid-deficient food should be used for feeding. Each mouse is prepared by tail vein injection of Example 2 18 The F-labeled tumor imaging agent is compound 9, the injection dose is 10μCi / 100μL (normal saline), and it is executed 1h and 2h after administration, and the blood, heart, liver, lung, kidney and other major organs are collected, weighed and counted . In the folate-suppressed control group, after injection 18 The F-labeled tumor imaging agent was injected with 100 μg folic acid 10 minutes before inhibition, and the mice were sacrificed 2 hours after the administration, and the main organs were also weighed and counted. The results are detailed in Tables 1 and 2.
[0062] It can be seen from Table 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com