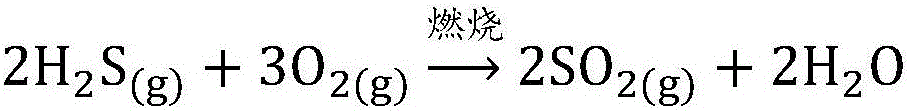
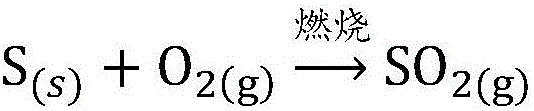SO2 absorbing and converting technological method
A process method, SO2 technology, applied in chemical instruments and methods, separation methods, inorganic chemistry, etc., can solve liquid-liquid heterogeneous system separation problems, difficult operation, electrode deactivation operation, etc., to solve fatal defects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
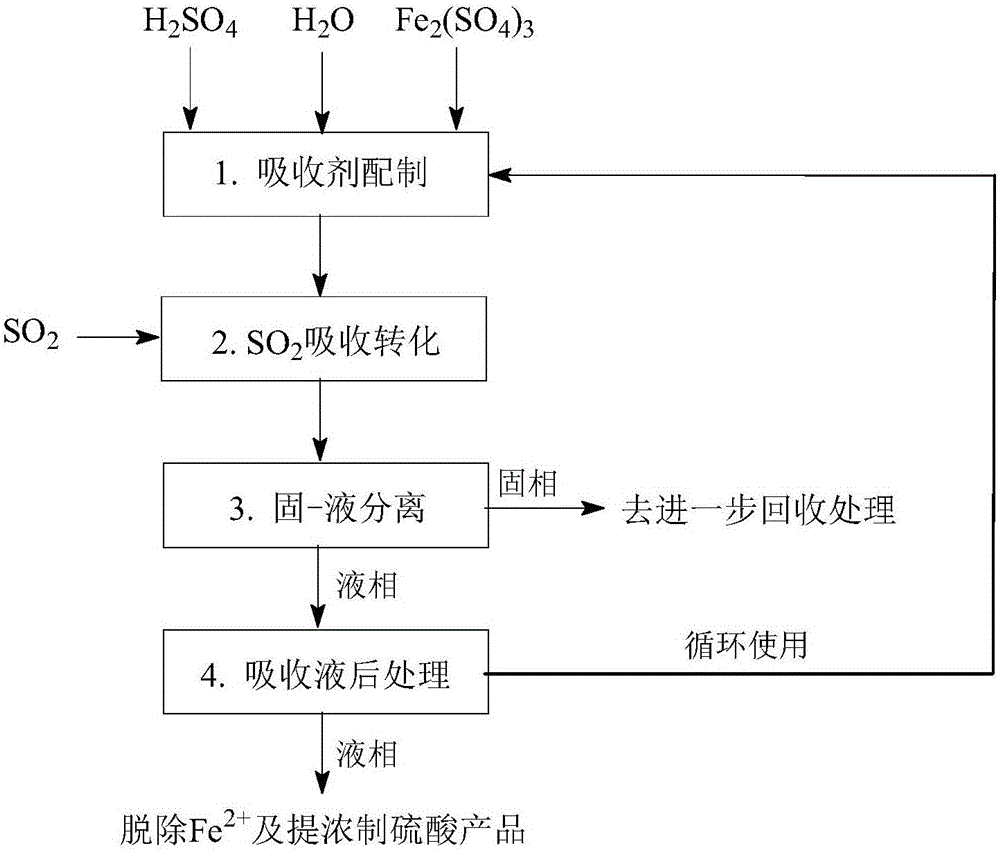
[0085] like figure 1 As shown, a SO 2 The processing method of absorption transformation, concrete steps are as follows:
[0086] (1) Absorbent preparation: In the absorbent preparation equipment, sulfuric acid and water are mixed to obtain an aqueous solution of sulfuric acid, and then ferric sulfate is dissolved in the aqueous solution of sulfuric acid to obtain a solution containing 0.2mol / L Fe 3+ and 0.2mol / LH 2 SO 4 Absorbent, the temperature is 20 ℃.
[0087] (2) SO 2 Absorption conversion: in the injection tower SO 2 In the absorption unit, the operating temperature is 20°C, and the Fe-containing 3+ Aqueous sulfuric acid solution as absorbent, Fe 3+ As an oxidizing agent, the Fe-containing 3+ Aqueous sulfuric acid solution as absorbent, Fe 3+ As an oxidizing agent, the SO 2 Absorb dissolved in sulfuric acid aqueous solution, absorb dissolved SO 2 with Fe 3+ The reaction converts to H 2 SO 4 , while Fe 3+ is reduced to Fe 2+ .
[0088] (3) Solid-liquid s...
Embodiment 2
[0091] like figure 1 As shown, a SO 2 The processing method of absorption transformation, concrete steps are as follows:
[0092] (1) Absorbent preparation: In the absorbent preparation equipment, Fe 2+ The sulfuric acid aqueous solution is mixed with water, and then dissolved in ferric sulfate to obtain 0.2mol H 2 SO 4 , 2.0mol / L Fe 3+ and 0.2mol / LFe 2+ absorbent at a temperature of 60°C.
[0093] (2) SO 2 Absorption transformation: in the spray tower SO 2 In the absorption unit, the operating temperature is 60°C, and the Fe-containing 3+ Aqueous sulfuric acid solution as absorbent, Fe 3+ As an oxidizing agent, the SO 2 Absorb dissolved in sulfuric acid aqueous solution, absorb dissolved SO 2 with Fe 3+ The reaction converts to H 2 SO 4 , while Fe 3+ is reduced to Fe 2+ .
[0094] (3) Solid-liquid separation: In the plate-and-frame filter press solid-liquid separation equipment, the material obtained in the previous step is subjected to solid-liquid separatio...
Embodiment 3
[0097] like figure 1 As shown, the specific steps are as follows:
[0098] (1) Absorbent preparation: In the absorbent preparation equipment, Fe 2+ The aqueous solution of sulfuric acid is mixed with water, and then dissolved in ferric sulfate to obtain 2.0mol / L H 2 SO 4 , 0.6mol / L Fe 3+ and 0.1mol / LFe 2+ absorbent at a temperature of 30°C.
[0099] (2) SO 2 Absorption conversion: in packed column SO 2 In the absorption device, the operating temperature is 30°C, and the Fe-containing 3+ Aqueous sulfuric acid solution as absorbent, Fe 3+ As an oxidizing agent, the SO 2 Absorb dissolved in sulfuric acid aqueous solution, absorb dissolved SO 2 with Fe 3+ The reaction converts to H 2 SO 4 , while Fe 3+ is reduced to Fe 2+ .
[0100] (3) Solid-liquid separation: In the solid-liquid separation equipment of the centrifuge, the material obtained in the previous step is subjected to solid-liquid separation to remove solid impurities to obtain Fe-containing 2+ aqueous s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com