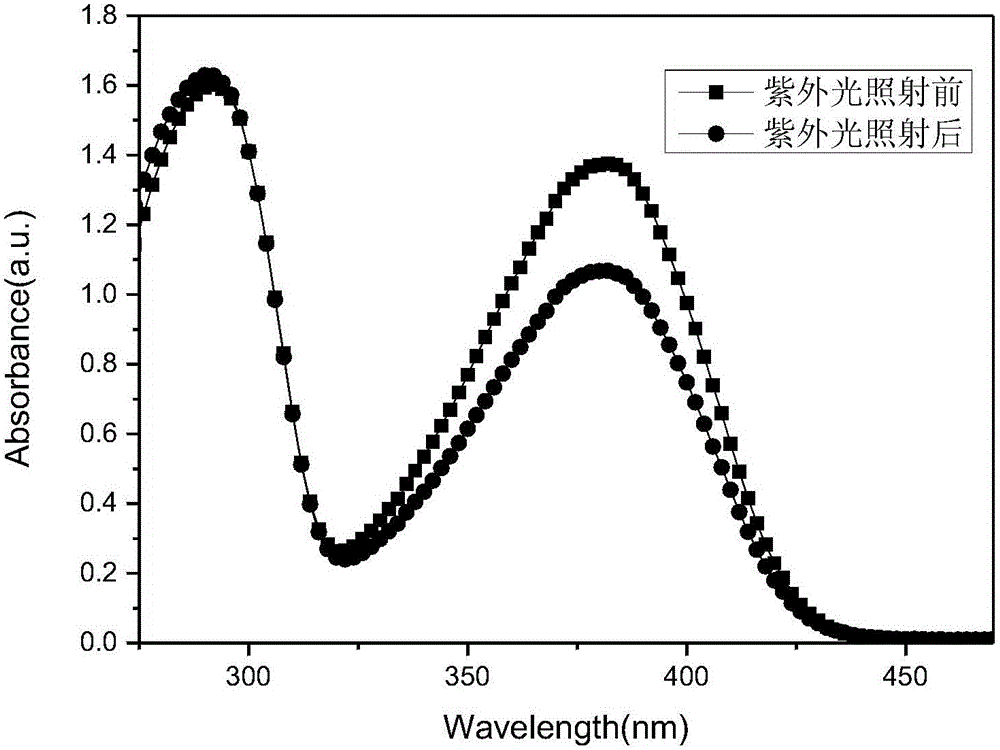
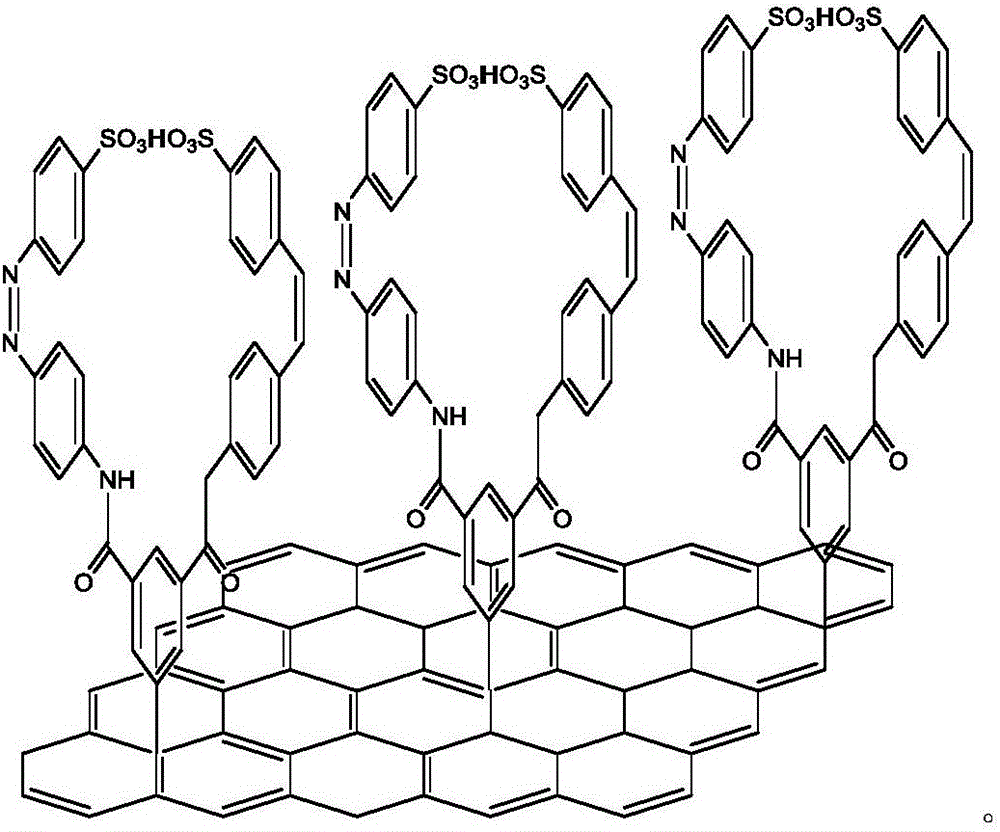
Double-branch azobenzene/graphene energy storage material and preparing method
A technology of double-branched azobenzene and energy storage materials, applied in the direction of sulfonic acid preparation, heat exchange materials, chemical instruments and methods, etc., can solve the problems of no amazing results, short half-life, limitations, etc. in the field of solar thermal storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention relates to the preparation method of double-branched azobenzene / graphene hybrid material, and its reaction process and explanation steps are as follows:
[0029]
[0030] 1) Amino protection: 5-aminoisophthalic acid is dissolved in N,N-dimethylformamide / water mixed solution of sodium hydroxide, wherein the molar ratio of 5-aminoisophthalic acid to sodium hydroxide is 1:1~1:1.5. Add the above solution dropwise to the di-tert-butyl dicarbonate solution in an ice-water bath, wherein the molar ratio of di-tert-butyl dicarbonate to 5-aminoisophthalic acid is 1:1; then naturally return to room temperature and react for 24~ 30 hours. The reaction product is first acidified with hydrochloric acid (the molar ratio to 5-aminoisophthalic acid is about 3:1 to 4:1, and then diluted with water to half to one third of the acidity. After that, the precipitate is filtered and washed with water and brine Repeated washing for 2 to 3 times. Finally, the solid was...
Embodiment 1
[0035] 1) Dissolve 1.087 g (12 mmol) of 5-aminoisophthalic acid in 30 ml of 1:1 N,N-dimethylformamide / water mixed solution containing 0.720 g of sodium hydroxide (18 mmol). In an ice-water bath, the above solution was added dropwise to a solution containing 2.619 g (12 mmol) of di-tert-butyl dicarbonate. Then naturally return to room temperature and react for 24 hours. The reaction product was acidified with 3M hydrochloric acid (5 mL) and then diluted with 30 mL of water. The precipitate was then filtered and washed twice with water and brine. Finally, product I was obtained by rotary evaporation and drying.
[0036]2) ① Add 1.096 milliliters (12.432 mmol) of thionyl chloride dropwise to a solution of 1.405 g of product I in 40 milliliters of dichloromethane. Then, 250 microliters of N,N-dimethylformamide was added dropwise to the above solution, and reacted at room temperature for 4 hours. The reaction product was then concentrated by distillation under reduced pressure....
Embodiment 2
[0040] 1) Dissolve 1.087 g (12 mmol) of 5-aminoisophthalic acid in 30 ml of 1:1 N,N-dimethylformamide / water mixed solution containing 0.520 g of sodium hydroxide (13 mmol). In an ice-water bath, the above solution was added dropwise to a solution containing 2.728 g (12.5 mmol) of di-tert-butyl dicarbonate. Then naturally return to room temperature and react for 25 hours. The reaction product was acidified with 3M hydrochloric acid (5 mL) and then diluted with 30 mL of water. The precipitate was then filtered and washed repeatedly 3 times with water and brine. Finally, product I was obtained by rotary evaporation and drying.
[0041] 2) ① Add 1.096 milliliters (12.432 mmol) of thionyl chloride dropwise to 40 milliliters of dichloromethane solution containing 1.42 g of product I. Then, 250 microliters of N,N-dimethylformamide was added dropwise to the above solution, and reacted at room temperature for 4 hours. The reaction product was then concentrated by distillation under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com