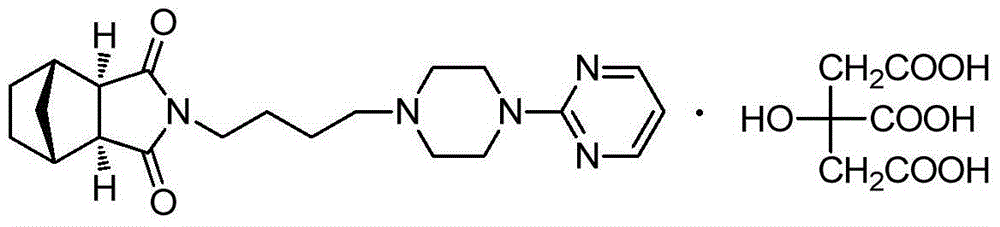
Tandospirone citrate compound
A technology of tandospirone citrate and tandospirone, which is applied in the field of preparation of the compound crystals, can solve the problems of less research on the crystal form of tandospirone citrate, unsatisfactory dissolution rate of preparations, and ineffectiveness. Ensure the effect of medication and other issues, to achieve the effect of ensuring the effect of medication, good dissolution effect, and improving bioavailability and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of Crystal Form IV of Tandospirone Citrate Compound
[0036] Get 41g citric acid and dissolve in 300mL acetone to make the acetone solution of citric acid. Weigh 80g tandospirone, add a mixed solution of 600mL acetone and 300mL ether, dissolve, add dropwise the prepared acetone citrate solution, heat and stir until the reaction is complete, naturally cool to room temperature and let stand for 6h, suction filter, wash , and dried under reduced pressure to obtain 118.8 g of white powdery tandospirone citrate crystal form IV, with a yield of 99.0%. Mass spectrum showed its ESI m / z: 383.
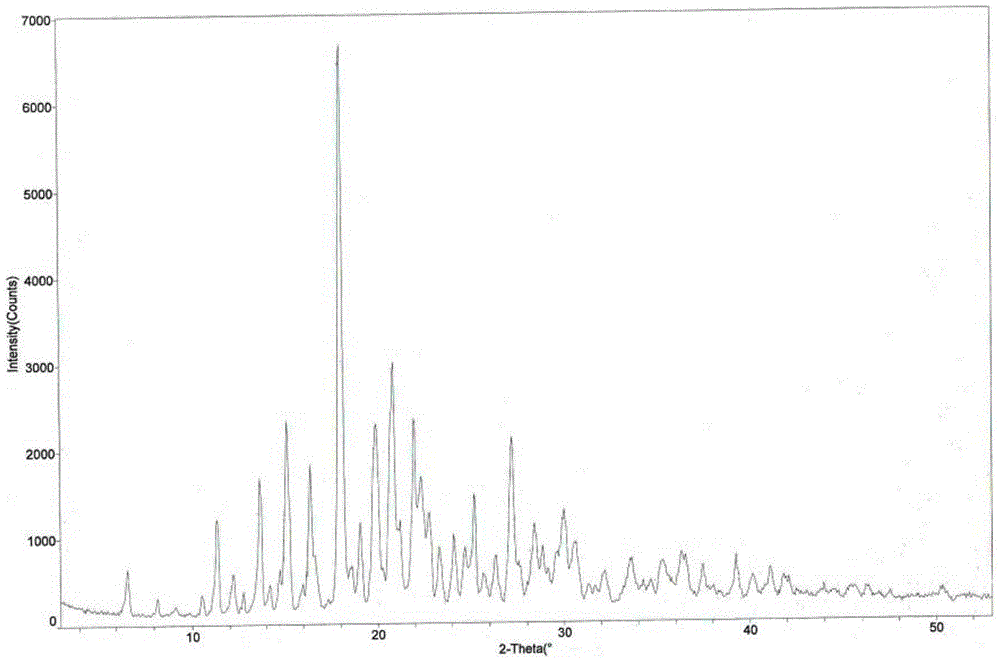
[0037] The melting point of tandospirone citrate crystal form IV was measured to be 165.5-166.5°C. Using Cu Kα radiation, the X-ray powder diffraction pattern of tandospirone citrate crystal form IV is measured in figure 1 , and its main relevant diffraction data are shown in Table 1 (2θ measurement error is ±0.2).
[0038] Table 1 X-ray powder diffraction data o...
Embodiment 2
[0040] Example 2 Preparation of tandospirone citrate compound crystal form IV
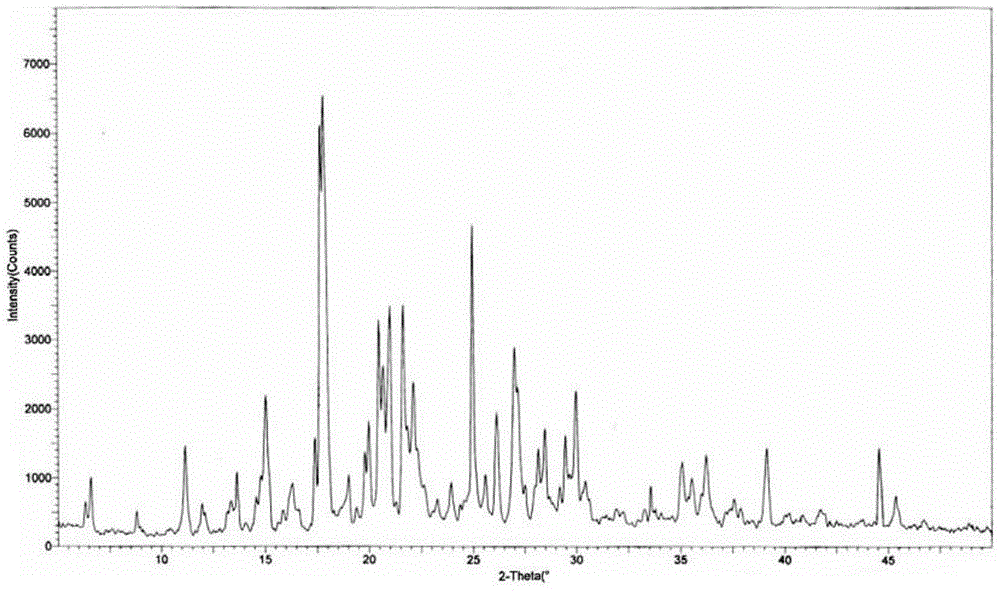
[0041] Get 40.1g of citric acid and dissolve in 250mL of acetone to make the acetone solution of citric acid. Weigh 80g of tandospirone, add 500mL of acetone and 50mL of diethyl ether mixed solution, wait until it is completely dissolved, add dropwise the prepared citric acid acetone solution, stir at 35°C until the reaction is complete, stop heating, and cool to room temperature naturally Stand still for 16 hours, filter with suction, wash, and dry under reduced pressure to obtain 118.6 g of white powdery crystal form IV of tandospirone citrate, with a yield of 98.8%. The structural analysis results and X-ray powder diffraction patterns of the obtained product are not significantly different from those in Example 1.
Embodiment 3
[0042] Example 3 Preparation of Crystal Form IV of Tandospirone Citrate Compound
[0043] Get 60.2g of citric acid and dissolve in 500mL of acetone to make the acetone solution of citric acid. Weigh 80g of tandospirone, add 1900mL of acetone and 400mL of diethyl ether mixed solution, until it is completely dissolved, add the prepared acetone citrate solution dropwise, stir at 40°C until the reaction is complete, stop heating, and cool to room temperature naturally Stand still for 2 hours, filter with suction, wash, and dry under reduced pressure to obtain 118.2 g of tandospirone citrate crystal form IV in the form of white powder, with a yield of 98.5%. The structural analysis results and X-ray powder diffraction patterns of the obtained product are not significantly different from those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com