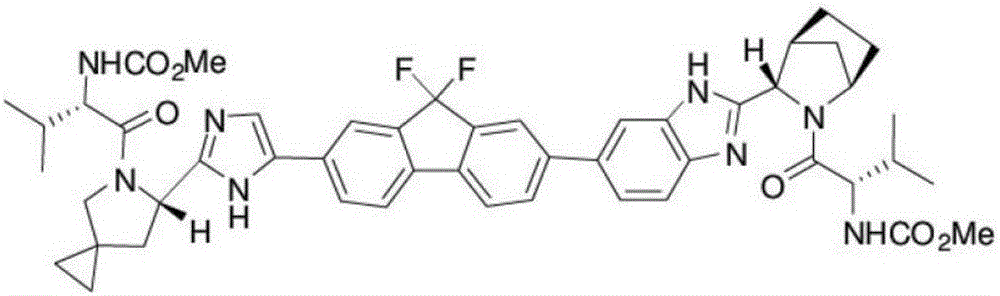
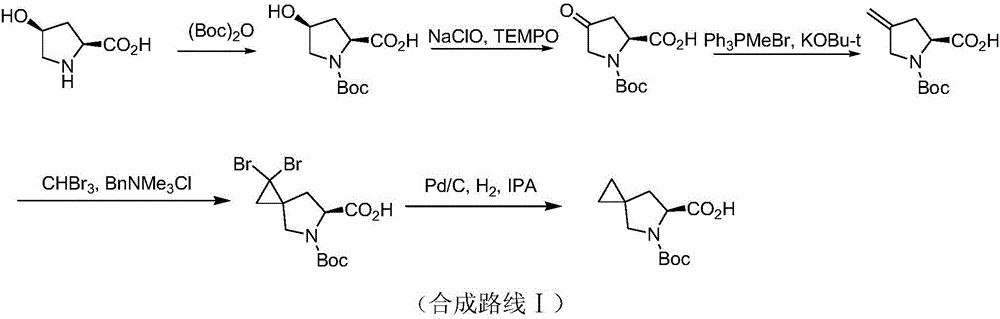
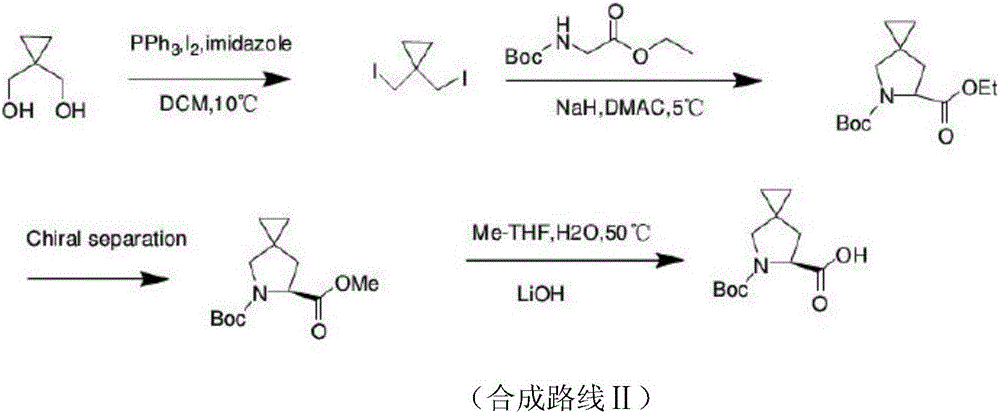
New method for synthesizing Ledipasvir chiral intermediate
A technology for chiral intermediates and synthesis methods, which is applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of complex drug structure, single synthesis route, and increased synthesis cost, and achieves simple process, high yield, and production cycle. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: A new method for synthesizing the chiral intermediate of Ledipavir as S-5-azaspirocyclo[2,4]heptane-6-carboxylic acid
[0053] The specific synthesis method includes the following steps:
[0054] S1. A compound of formula A is prepared by ring closure of 1,1-dihalomethylcyclopropane and N-Boc-glycine ethyl ester in a basic reaction solvent; wherein, the reaction solvent is an amide solvent; the base It is potassium alkoxide; the reaction temperature is -10℃;
[0055] S2. The compound of formula A is saponified and hydrolyzed, and the BOC protection is removed to obtain 5-azaspirocyclo[2,4]heptane-6-carboxylic acid racemate; the saponification and hydrolysis is carried out in an alkaline solvent. The solvent is alcohols, the alkali is sodium hydroxide; the acid used for the BOC deprotection is hydrochloric acid; the reaction temperature is 0°C;
[0056] S3.5-azaspirocyclo[2,4]heptane-6-carboxylic acid racemate was asymmetrically resolved to prepare S-5-azaspirocyclo[...
Embodiment 2
[0057] Example 2: A new method for synthesizing the chiral intermediate of Ledipavir as S-5-azaspirocyclo[2,4]heptane-6-carboxylic acid
[0058] The specific synthesis method includes the following steps:
[0059] S1. Ring-closure of 1,1-dihalomethylcyclopropane and N-Boc-glycine ethyl ester in a basic reaction solvent to prepare a compound of formula A; wherein, the reaction solvent is an ether solvent; the base It is sodium alkoxide; the reaction temperature is 100℃;
[0060] S2. The compound of formula A is saponified and hydrolyzed, and the BOC protection is removed to obtain 5-azaspirocyclo[2,4]heptane-6-carboxylic acid racemate; the saponification and hydrolysis is carried out in an alkaline solvent. The solvent is ethers, the alkali is potassium hydroxide; the acid used in the de-BOC protection is sulfuric acid; the reaction temperature is 100°C;
[0061] S3.5-azaspirocyclo[2,4]heptane-6-carboxylic acid racemate was asymmetrically resolved to prepare S-5-azaspirocyclo[2,4]hept...
Embodiment 3
[0062] Example 3: A new method for synthesizing the chiral intermediate of Ledipavir as S-5-azaspirocyclo[2,4]heptane-6-carboxylic acid
[0063] The specific synthesis method includes the following steps:
[0064] S1. A compound of formula A is prepared by ring closure of 1,1-dihalomethylcyclopropane and N-Boc-glycine ethyl ester in a basic reaction solvent; wherein, the reaction solvent is an amide solvent; the base It is sodium hydride; the reaction temperature is 45°C;
[0065] S2. The compound of formula A is saponified and hydrolyzed, and the BOC protection is removed to obtain 5-azaspirocyclo[2,4]heptane-6-carboxylic acid racemate; the saponification and hydrolysis is carried out in an alkaline solvent. The solvent is alcohols, the alkali is strong sodium oxide; the acid used for the BOC protection is phosphoric acid; the reaction temperature is 38°C;
[0066] S3.5-azaspirocyclo[2,4]heptane-6-carboxylic acid racemate was asymmetrically resolved to prepare S-5-azaspirocyclo[2,4]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com