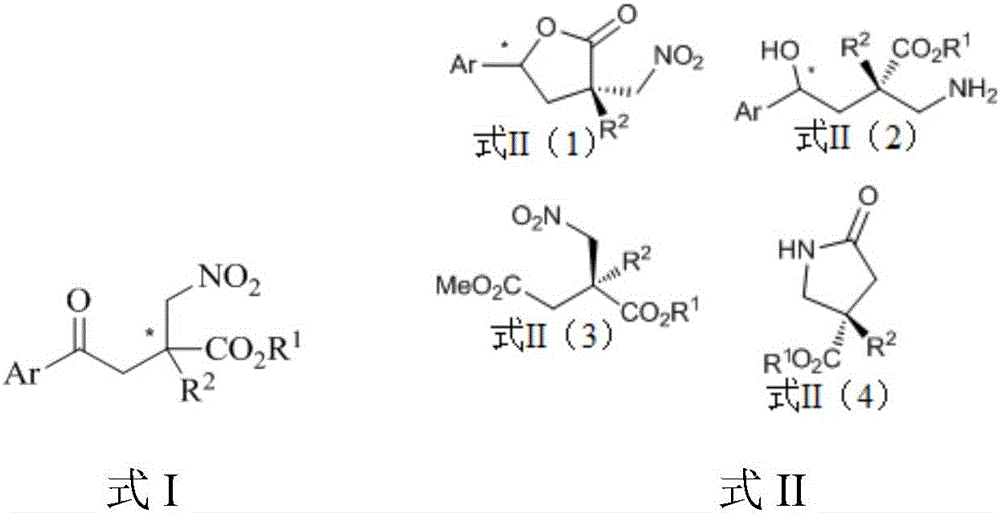
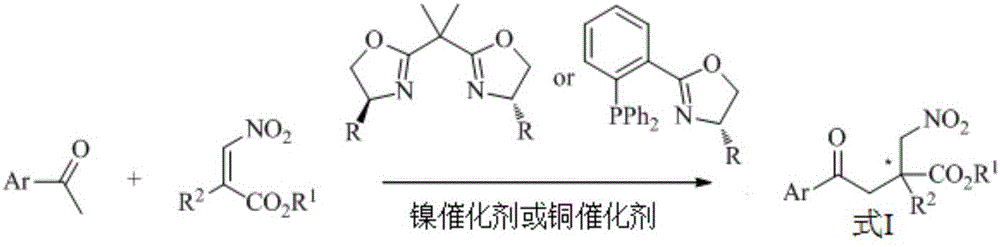
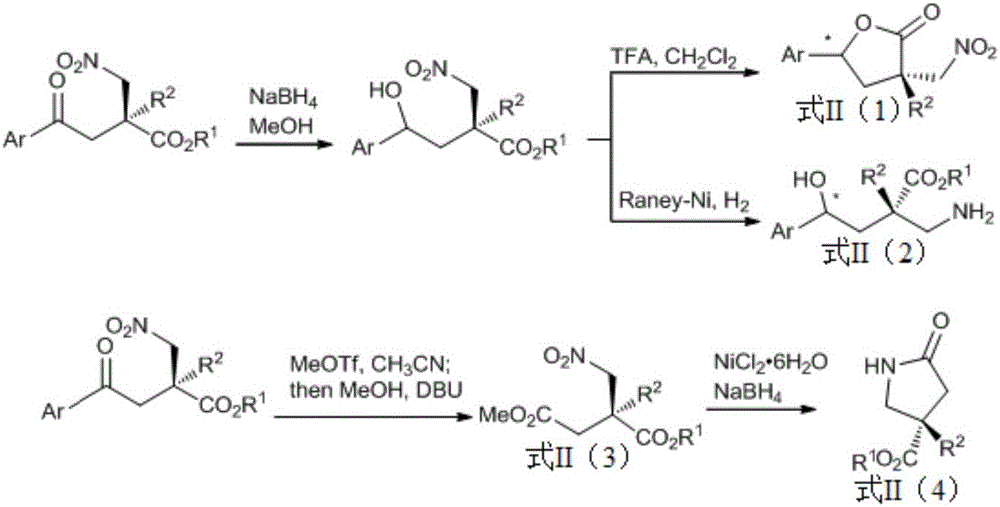
Preparation of alpha,alpha-disubstituted-beta-nitro ester compound containing full-carbon quaternary carbon chiral center and nitrogen aromatic heterocyclic ring and derivatives thereof
A chiral center, aromatic heterocycle technology, applied in the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., can solve problems such as steric hindrance difficult to generate, and achieve high yield, high optical purity, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Synthesis of (+)-2-nitromethyl-2-aryl-4-(azaaromatic ring-2-yl)-4-butanone ester
[0026] Under the protection of nitrogen atmosphere and room temperature, add bisoxazoline chiral ligand (R is phenyl)-Ni(acac) in isopropanol 2 Or phosphine-oxazoline chiral ligand (R is tert-butyl)-Cu(OAc) 2 .H 2 O, then add 2-acetyl azaaromatic hydrocarbon, after stirring at room temperature for 15 minutes, then add α-substituted-β-nitroacrylate, and continue to stir at room temperature for reaction; after the reaction is completed, the solvent is removed, and purified by silica gel column chromatography to obtain (+)-2-Nitromethyl-2-aryl-4-(azaaromatic ring-2-yl)-4-butanone ester, which contains all-carbon quaternary carbon chiral center and nitrogen aromatic heterocycle α,α-Disubstituted-β-nitroester compounds, the yield and photochemical purity of the reaction are shown in Table 1. It can be seen from Table 1 that the yield of the reaction is high (85%-97%) and the o...
Embodiment 2
[0030] Embodiment 2: Synthesis of (+)-2-nitromethyl-2-phenyl-4-(pyridin-2-yl)-4-oxobutanoic acid tert-butyl ester
[0031]
[0032] To a dry 10 mL round bottom flask was added the bisoxazoline chiral ligand (8.0 mg, 0.024 mmol), Ni(acac) 2 (5.1mg, 0.020mmol), add isopropanol 2mL under nitrogen protection, stir at room temperature for 1.5h, then add 2-acetylpyridine (24.2mg, 0.20mmol), stir for 15min, then add 3-nitro-2-phenyl tert-Butyl acrylate (75.1 mg, 0.30 mmol). After adding the sample, react overnight at room temperature; TLC detects that the reaction is complete, and the reaction mixture is separated and purified by silica gel chromatography (ethyl acetate:petroleum ether=1:10, volume ratio) after desolvation under reduced pressure to obtain a white solid ( +)-tert-butyl 2-nitromethyl-2-phenyl-4-(pyridin-2-yl)-4-oxobutanoate. The yield of reaction is 95%; (c=1.0, CH 2 Cl 2 ), the optical purity is 97% ee.
[0033] The white solid was analyzed and identified by...
Embodiment 3
[0034] Embodiment 3: Synthesis of (+)-2-nitromethyl-2-phenyl-4-(quinolin-2-yl)-4-oxobutanoic acid tert-butyl ester
[0035]
[0036]To a dry 10 mL round bottom flask was added the bisoxazoline chiral ligand (8.1 mg, 0.024 mmol), Ni(acac) 2 (5.1mg, 0.020mmol), add isopropanol 2mL under nitrogen protection, stir at room temperature for 1.5h, then add 2-acetylquinoline (34.2mg, 0.2mmol), stir for 15min and then add 3-nitro-2- Tert-butyl phenylacrylate (75.1mg, 0.3mmol), after adding the sample, react overnight at room temperature; TLC detects that the reaction is complete, and the reaction mixture is separated and purified by silica gel chromatography directly after decompression precipitation (ethyl acetate: petroleum Ether = 1:10, volume ratio), to obtain tert-butyl (+)-2-nitromethyl-2-phenyl-4-(quinolin-2-yl)-4-oxobutanoate as a white solid. The yield of reaction is 97%; (c=0.55, CH 2 Cl 2 ), the photochemical purity is 98% ee.
[0037] The white solid was analyzed an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com