Method for preparing cathode material lithium nickel cobalt aluminate for lithium ion battery by spray drying
A lithium-nickel-cobalt-aluminate and lithium-ion battery technology, which is applied to battery electrodes, circuits, electrical components, etc., can solve problems such as unsuitability for large-scale industrial production, difficult simultaneous homogeneous precipitation of nickel, cobalt and aluminum, and increased costs. Improved cycle rate performance, better product controllability, and increased production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
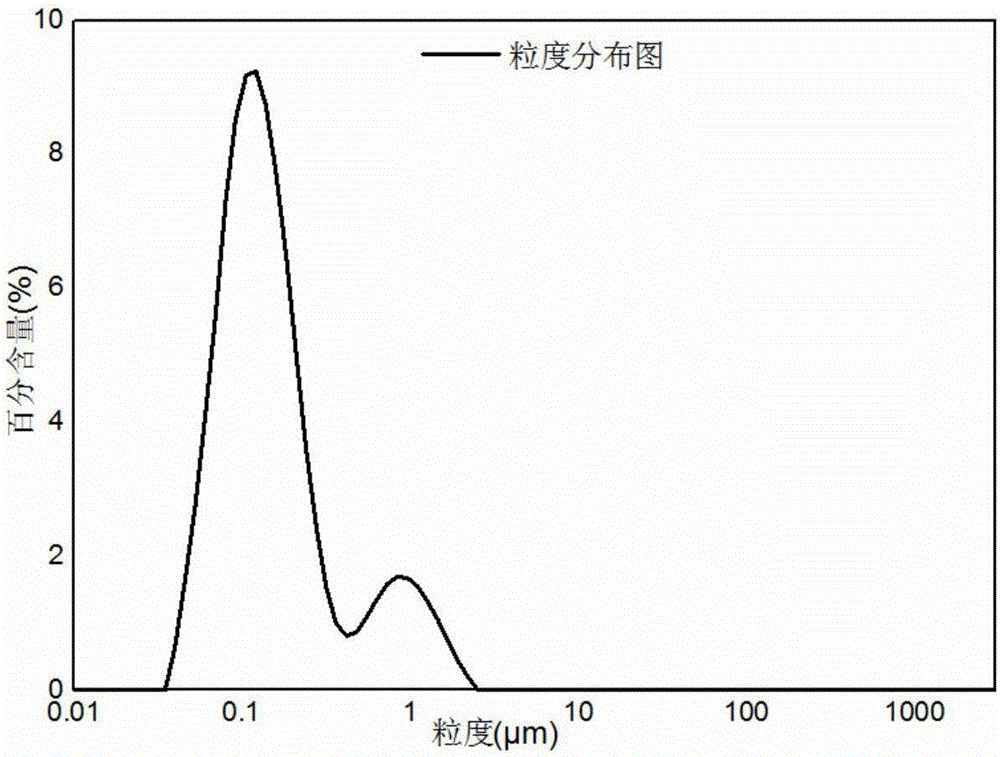
[0034] According to the molar ratio of Ni:Co:Al is 0.8:0.15:0.05, take nickel hydroxide, cobalt oxyhydroxide, aluminum oxide and aluminum fluoride and mix them, then according to the molar ratio Li:(Ni+Co+Al)=1.05 :1 Add lithium hydroxide and mix, add deionized water to the mixed material according to the liquid-solid mass ratio of 0.25 for sand milling, the diameter of zirconium balls used for sand milling is 0.05mm, the main engine speed is 2000r / min, and the sand milling time is 3.5h , D50 of the slurry after sanding is 0.105 μm.
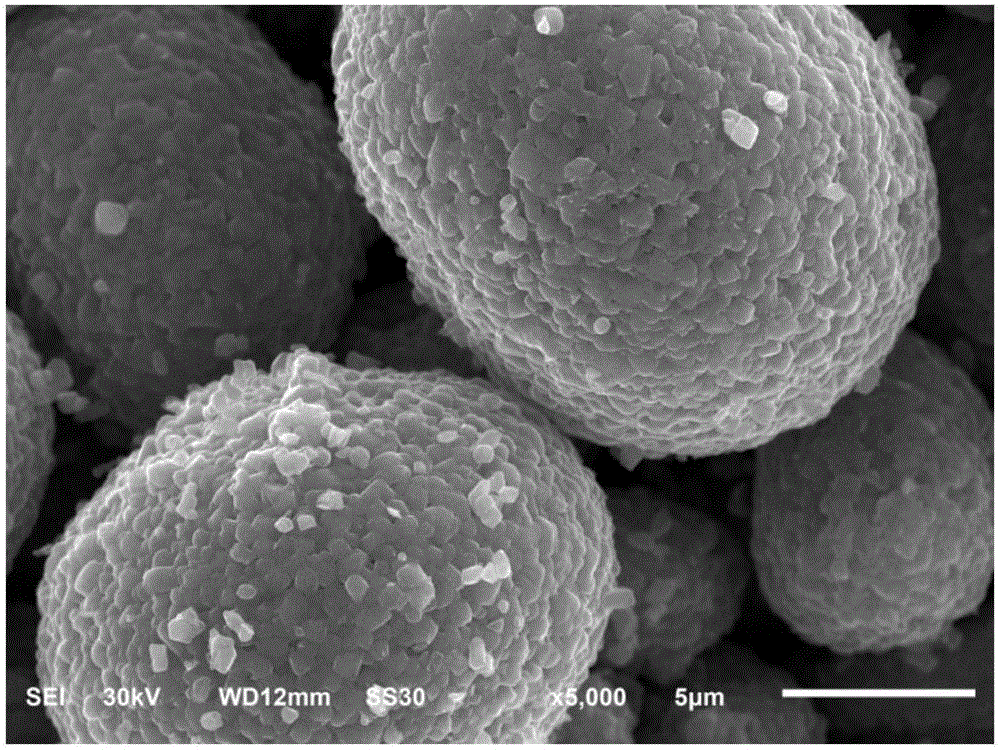
[0035] The sanded slurry is transferred to a turnover drum with stirring for spray drying. By adjusting the feed rate of 600ml / h, the inlet pressure of 0.65MPa, the temperature near the nozzle of 220°C, and the outlet temperature of 100°C, the D50 is 8.570μm The nickel cobalt lithium aluminate precursor.
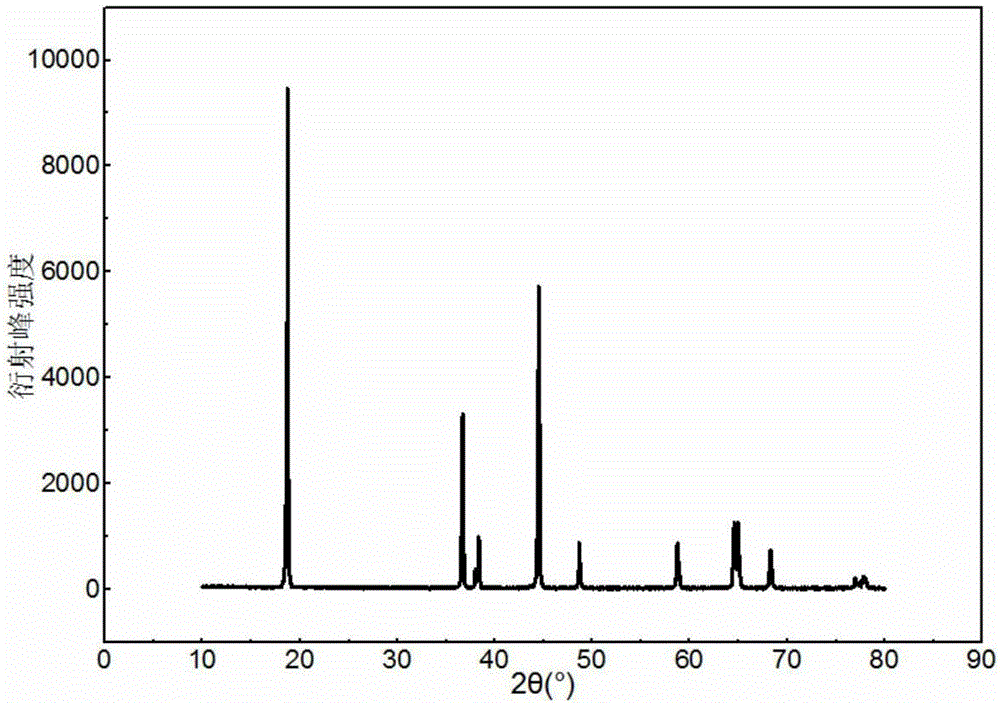
[0036] Sinter the above nickel-cobalt-lithium-aluminate precursor in two stages. First, sinter at 500°C for 6 hours under oxygen flow, then r...
Embodiment 2
[0039]Weigh nickel carbonate, cobalt carbonate and aluminum sesquioxide according to the molar ratio of Ni:Co:Al being 0.85:0.1:0.05, and then add lithium hydroxide according to the molar ratio Li:(Ni+Co+Al)=1.08:1 Mixing, according to the liquid-solid mass ratio of 0.30, add deionized water to the mixed material for sanding, the diameter of the zirconium ball used for sanding is 0.1mm, the main engine speed is 1800r / min, the sanding time is 3h, and the slurry after sanding is D50 is 0.132 μm.
[0040] The sanded slurry is transferred to a turnover drum with stirring for spray drying. By adjusting the feed rate of 800ml / h, the inlet pressure of 0.45MPa, the temperature near the nozzle of 250°C, and the outlet temperature of 105°C, the D50 is 11.621μm The nickel cobalt lithium aluminate precursor.
[0041] Sinter the above-mentioned nickel-cobalt-lithium aluminate precursor in two stages. Firstly, it is sintered at 550°C for 8 hours under an oxygen flow condition, and then the...
Embodiment 3
[0044] Weigh nickel hydroxide, cobalt carbonate, aluminum oxide and aluminum phosphate according to the molar ratio of Ni:Co:Al being 0.75:0.2:0.05, and then mix according to the molar ratio Li:(Ni+Co+Al)=1.02:1 Add lithium hydroxide and mix, and add deionized water to the mixed material according to the liquid-solid mass ratio of 0.20 for sand grinding. The D50 of the milled slurry was 0.086 μm.
[0045] The sanded slurry is transferred to a turnover drum with stirring for spray drying. By adjusting the feed rate of 300ml / h, the inlet pressure of 0.75MPa, the temperature near the nozzle of 190°C, and the outlet temperature of 95°C, the D50 is 5.023μm The nickel cobalt lithium aluminate precursor.
[0046] Sinter the above nickel-cobalt-lithium-aluminate precursor in two stages. First, sinter at 450°C for 8 hours under oxygen flow, then raise the sintering temperature to 780°C, and sinter in oxygen flow for 18 hours. After sintering, cool with the furnace. Lithium-ion batter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| D50 | aaaaa | aaaaa |
| D50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com