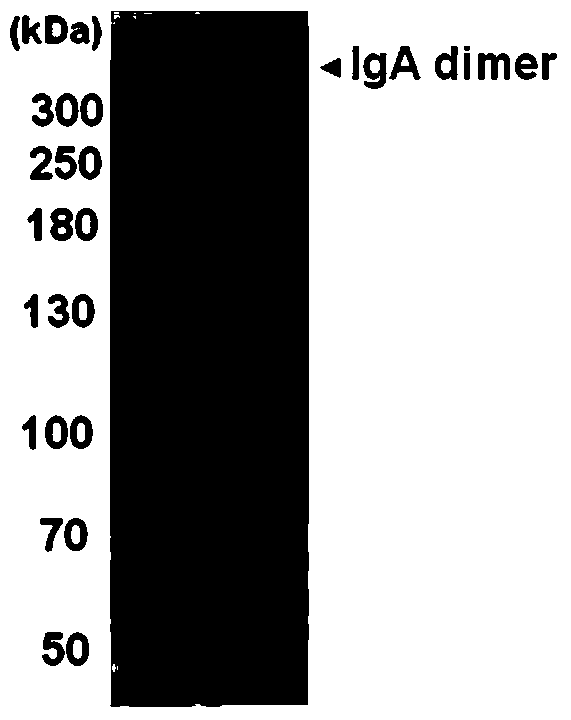
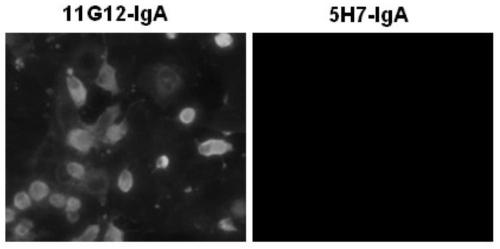
An anti-enterovirus ev71 IgA monoclonal antibody and its application
A monoclonal antibody, EV71 technology, applied in the field of biotechnology and immunology, can solve the problem of inability to induce immune protection, and achieve the effect of improving specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Obtaining of hybridoma cells secreting anti-enterovirus EV71IgA monoclonal antibody:
[0038] 1.1. Antigen preparation
[0039] The complete VP1 gene of EV71BrCr strain was cloned into the vector pET28a. Briefly, Escherichia coli BL21(DE3) containing recombinant 3D expression vectors were grown, induced to produce these recombinant proteins, and purified by affinity chromatography on Ni-NTA columns (Qiagen).
[0040] 1.2. Immunization and preparation of EV71VP1-specific IgG monoclonal antibody
[0041] Methods for preparing EV71VP1-specific monoclonal antibodies such as (Li YM, Liu F, Han C and YanHM. Monoclonal antibody that blocks the Toll-like receptor 5 binding region of flagellin. Hybridoma (Larchmt). 2012 Feb; 31 (1): 60-62). Briefly, 5-week-old female SPFBALB / c mice were immunized subcutaneously with 100 μg of VP1 at 2-week intervals. Four weeks after the last boost and 3 days before cell fusion, mice were boosted with 200 μg VP1 intraperitoneally. Three day...
Embodiment 2
[0054] Obtaining EV71 VP1-specific IgA monoclonal antibody:
[0055] 1. Preparation of mouse ascites
[0056] a.Prime: Each BALB / c mouse was intraperitoneally injected with 500 μl of liquid paraffin.
[0057] b. After feeding for 15 days, each mouse was intraperitoneally injected with 3-5×10 5 hybridoma cells in an injection volume of 1 ml.
[0058] c. Closely observe the physiological conditions of the mice. After the abdominal volume of the mice increases, the movement is inconvenient, and the vitality decreases (usually after 8-14 days), the mice are killed by cervical dislocation, the chest cavity is opened, and the glass straw is inserted from the diaphragm. Absorb ascites. The titer of ascites is 2 million.
[0059] 2. Silica degreasing
[0060] a. Centrifuge the freshly collected ascites at 2000r / min for 15 minutes to remove cell components and tissue debris, take the upper clear ascites, and add an equal volume of PBS with pH 7.2 to dilute;
[0061] b. Add 150 mg...
Embodiment 3
[0077] Application of IgA monoclonal antibody in the preparation of drugs for inhibiting enterovirus 71:
[0078] 1. Extracellular neutralization of 11G12-IgA
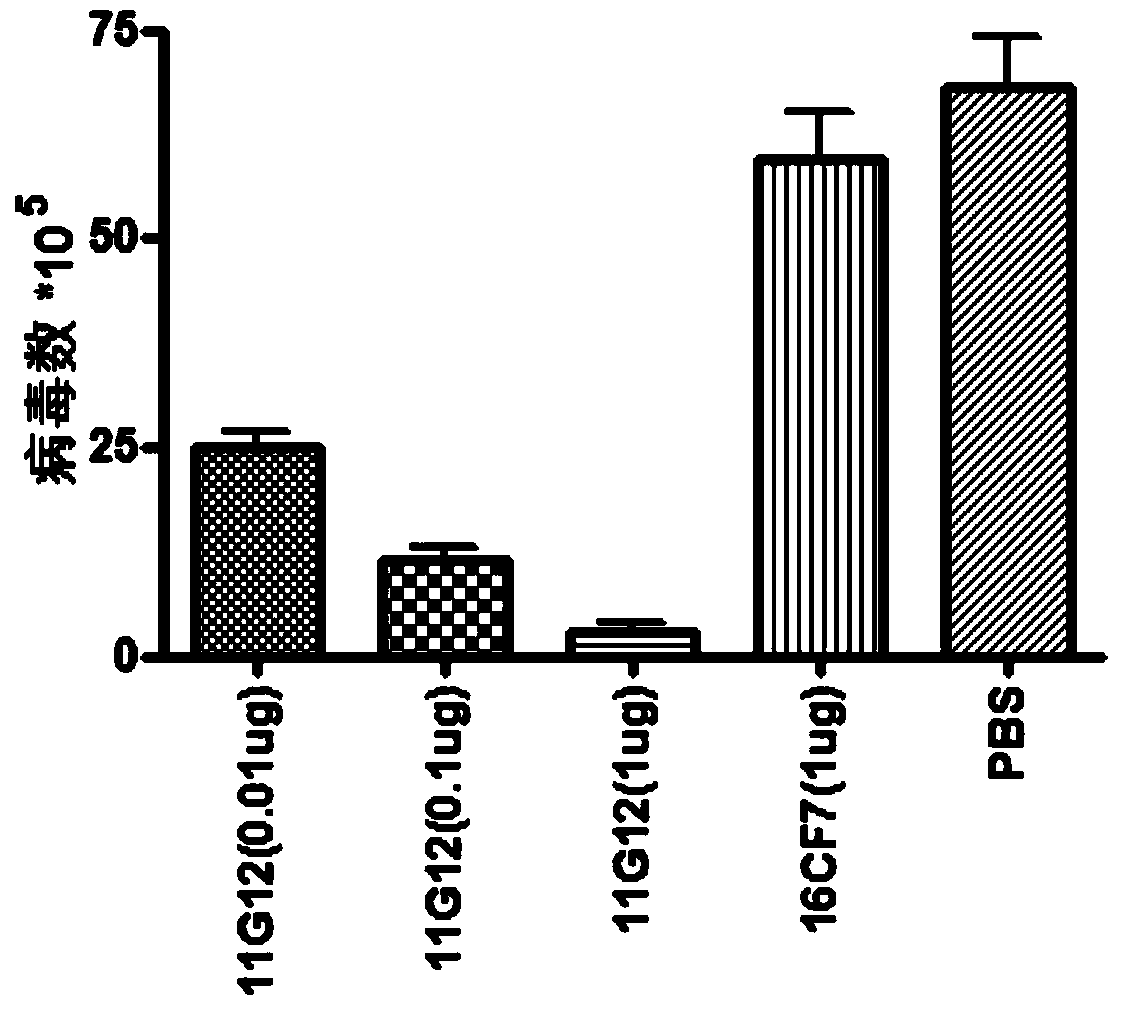
[0079] Will 1×10 4 PFU EV71 (BrCr strain) was added to serial dilutions of 200 μl monoclonal antibody 11G12-IgA (16CF7-IgA IgA antibody specific to measles virus matrix protein M served as an irrelevant antibody control). After 1 hour of incubation, the mixture was used to infect Caco-2 cells. EV71-infected Caco-2 cells were harvested and virus titers in these cell samples were determined by plaque assay. Such as image 3 As shown, at the concentration of 0.01-1 μg / well, the infection of the virus can be significantly reduced. 1ug / well (200ul) can inhibit 90% virus, 0.1ug / well can inhibit 70% virus infection, and 0.01ug / well can inhibit 50% virus infection.
[0080] 2. EV71 VP1-specific 11G12-IgA inhibits the replication of EV71 in cells
[0081] Spread Transwell with Vero C1008-pIgR cells, 2×10 5 One per hole, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com