Photocatalyst for producing hydrogen through photocatalytic water splitting and preparation method thereof
A water splitting and photocatalysis technology, applied in the chemical industry, can solve the problems of expensive equipment and poor catalytic effect of photocatalysts, and achieve the effects of improving utilization rate, facilitating large-scale promotion, and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
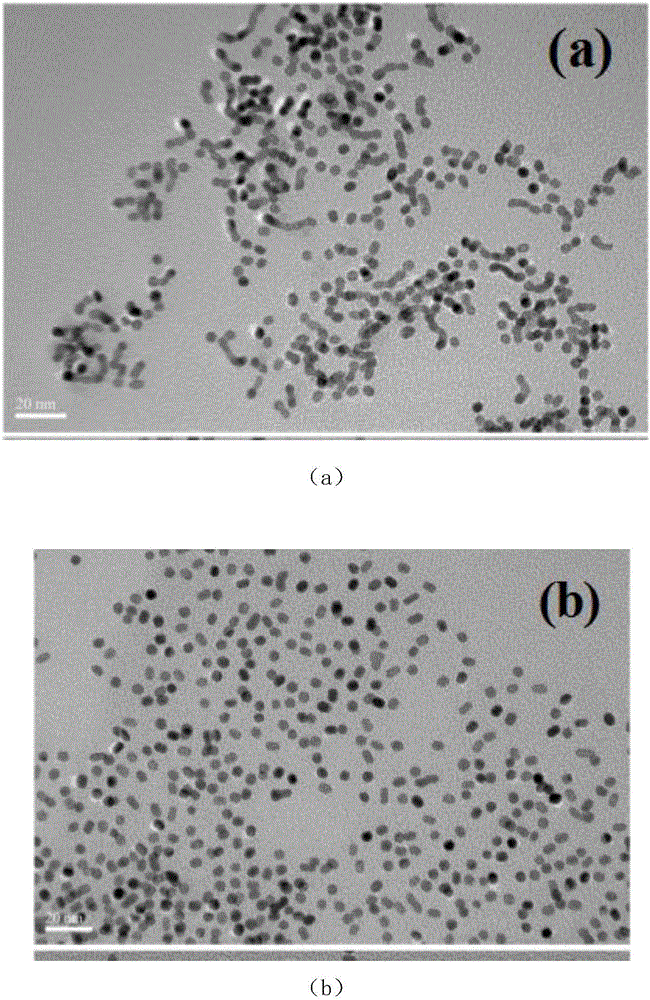
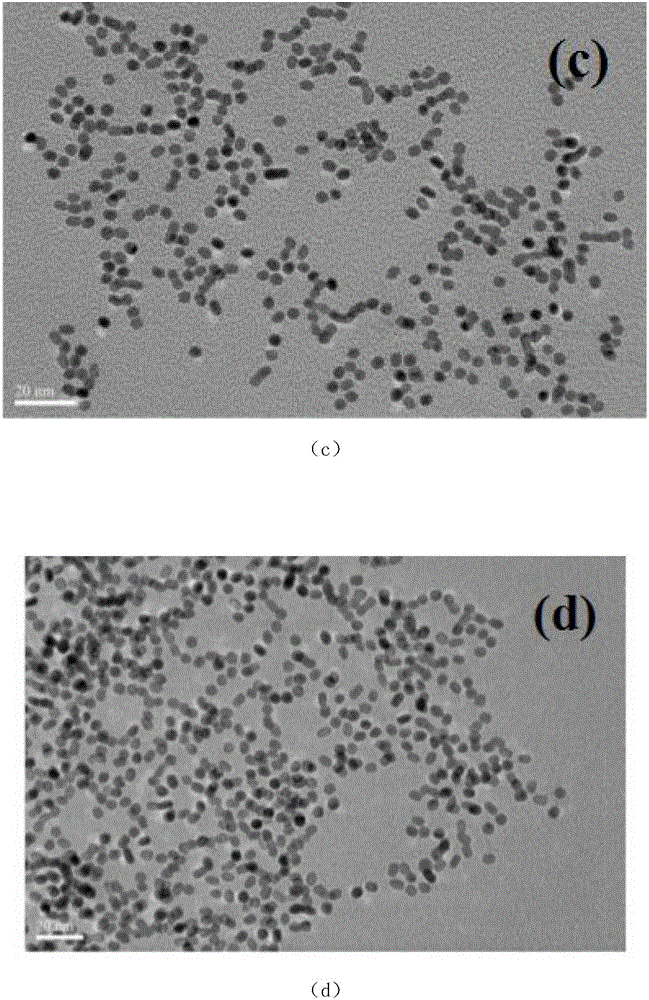
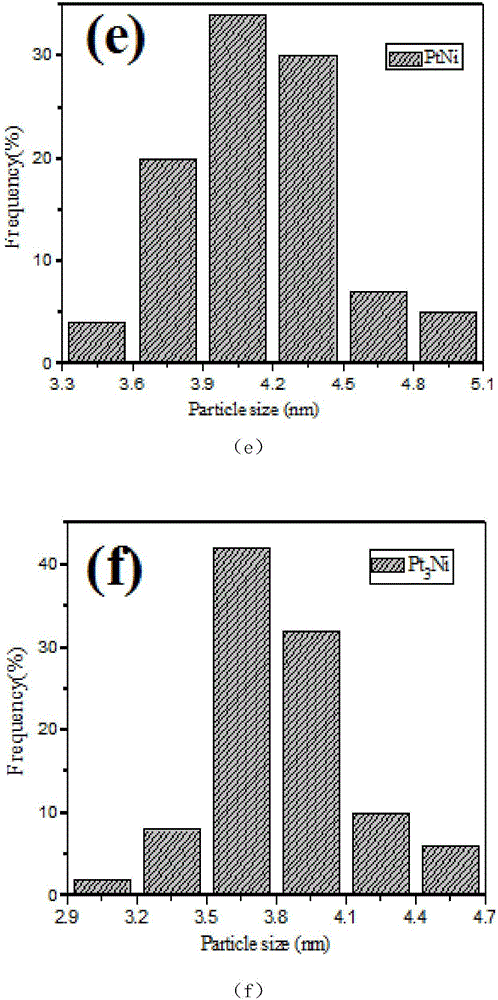
[0019] The preparation steps of the present invention are as follows: 1ml of potassium chloroplatinite (K 2 PtCl4 20.0Mm), 1ml of nickel acetylacetonate (Ni(acac) 2 20.0Mm) solution, 160mg polyvinylpyrrolidone (PVP) and 0.5mmol NaI were dissolved in 10ml of N,N-dimethylformamide solution, ultrasonically dispersed evenly, transferred to a 25mL autoclave, and the solution was heated at 150°C Under the condition of hydrothermal reaction for 5 hours. The product is cleaned with a mixed solution of ethanol and acetone (V / V=1:3) by ultrasonication, centrifugation at 8000 rpm, and NaBH 4 / TBA and then washed to obtain nanoparticles for the characterization test of the experiment. The mass ratio of Pt and Ni is 2:1, 3:1, 4:1, adding potassium chloroplatinite (K 2 The amount of PtCl4 20.0Mm) is 2ml, 3ml, 4ml.
[0020] The above-prepared unwashed Pt-Ni nanoparticles were loaded onto the commercial CdS surface uniformly dispersed by ultrasound, wherein the loading amount of Pt-Ni wa...
Embodiment 2
[0021] Embodiment 2 electrocatalytic hydrogen evolution performance test
[0022] The electrochemical test analysis is completed on the CHI660E electrochemical workstation, using a three-electrode test system. The preparation method of the working electrode: the unwashed Pt-Ni nanoparticles prepared above are added dropwise and deposited onto the surface of the nano-carbon powder uniformly dispersed by ultrasonic waves, wherein the loading of Pt-Ni is 20wt% of the nano-carbon powder, and kept stirring state for 2 hours, then the sample was subjected to suction filtration, NaBH4 / TBA cleaning, vacuum drying and grinding, and finally the PtNi / nano-carbon powder composite catalyst was prepared for electrocatalytic hydrogen evolution performance test, and 2mg of PtNi / nano-carbon powder was weighed , dissolved in 400 μL deionized water, 600 μL ethanol and 120 μL 5% Nafion solution, and ultrasonically dispersed for 10 minutes. Take 5 μL of the sample solution and drop it on the glas...
Embodiment 3
[0024] Example 3 Photocatalytic hydrogen production under visible light:
[0025] The light source of the photocatalytic activity test reaction is a 300W Xe lamp, and the infrared light part is removed by the cooling circulating water above the reactor, and the ultraviolet light part is removed by a 420nm filter. At room temperature, add a certain amount of deionized water, methanol as a sacrificial agent, and 0.05 g of CdS photocatalyst loaded with 0.5 wt% Pt-Ni into the reactor. After stirring for a while, connect the reactor to the hydrogen production reaction system by photolysis of water , to pump out the air in the system. Using visible light with a wavelength greater than 420nm as the light source, the hydrogen production was measured at regular intervals to study its photocatalytic activity.
[0026] like image 3 Shown is the visible-light photocatalytic activity rate of hydrogen production under the above conditions with the cocatalyst of Pt and Ni alloy nanopartic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com