Optimized gene of ietalurus punetaus LEAP-2 mature peptide and preparation method of recombinant protein of optimized gene
A channel catfish and LEAP-2 technology, applied in the field of genetic engineering, can solve the problems of reduced product activity, no optimization, unknown expression level, etc., to achieve the effects of increasing yield, saving costs, and facilitating later operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Recombinant expression of unoptimized native cDNA (SEQ ID NO:1) of channel catfish LEAP-2 mature peptide in Pichia pastoris GS115
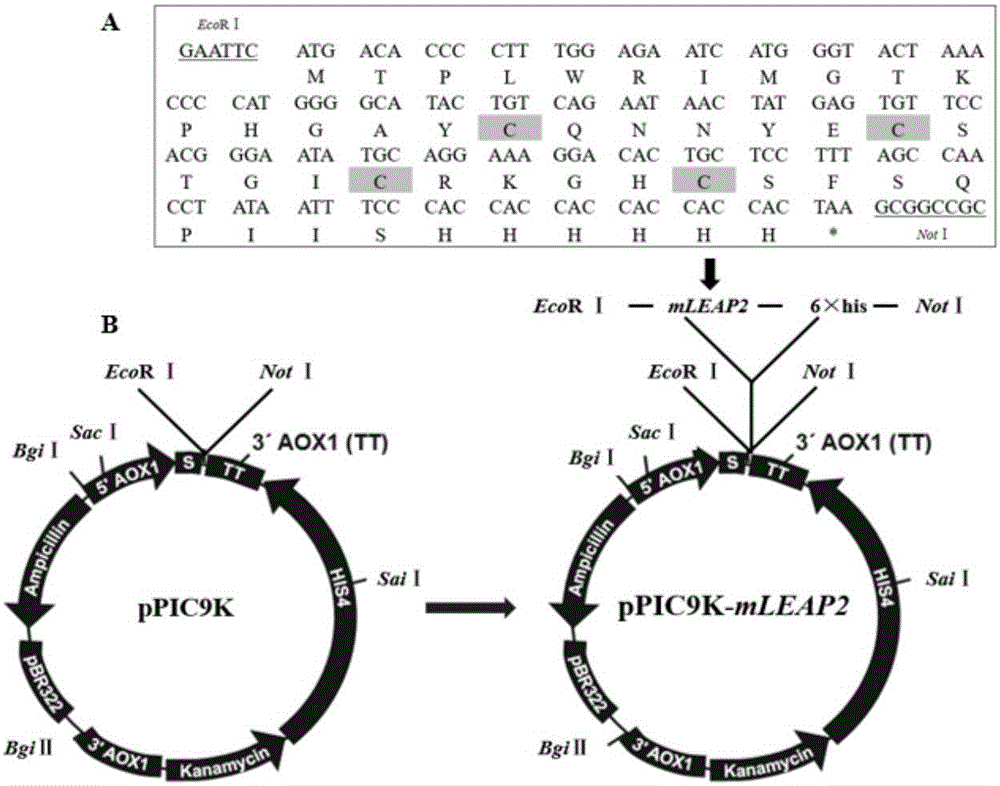
[0049] 1.1 Addition of restriction sites for channel catfish LEAP-2 mature peptide gene
[0050] Design 3 primers containing EcoRI, NotI restriction site and 6×His tag respectively (see Table 1). For the first PCR, the prokaryotic expression vector "pET-32a-mLEAP2" of the existing channel catfish LEAP-2 mature peptide gene "mLEAP2" was used as a template, and LF and LR1 were used as primers. The reaction system and conditions were as follows:
[0051] "pET-32a-mLEAP2" 2.5 μL, forward and reverse primers 4.0 μL (10 μmol / L), dNTP (10 mmol / L) 8.0 μL, PCR buffer 10 μL, Taq DNA polymerase (Takara, Otsu, Japan) 1 μL, with Sterile water was adjusted to 100 μL; 30 cycles of pre-denaturation at 94°C for 3 min, denaturation at 94°C for 30 s, annealing at 54.6°C for 30 s, extension at 72°C for 1 min, and a final extension at 72°C for 5 min. Using th...
Embodiment 2
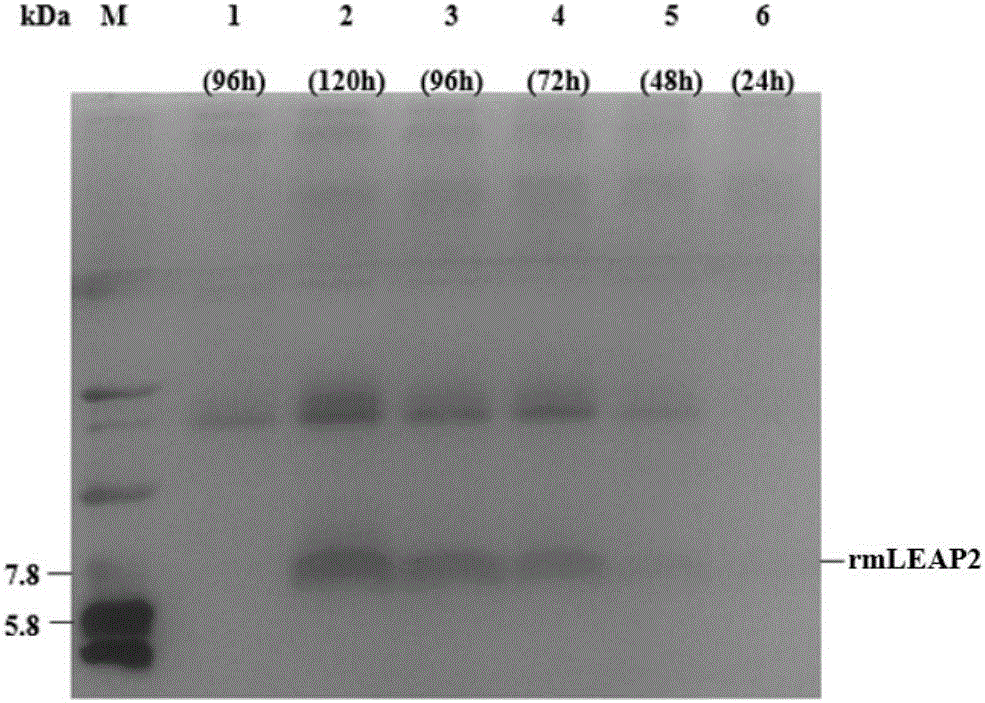
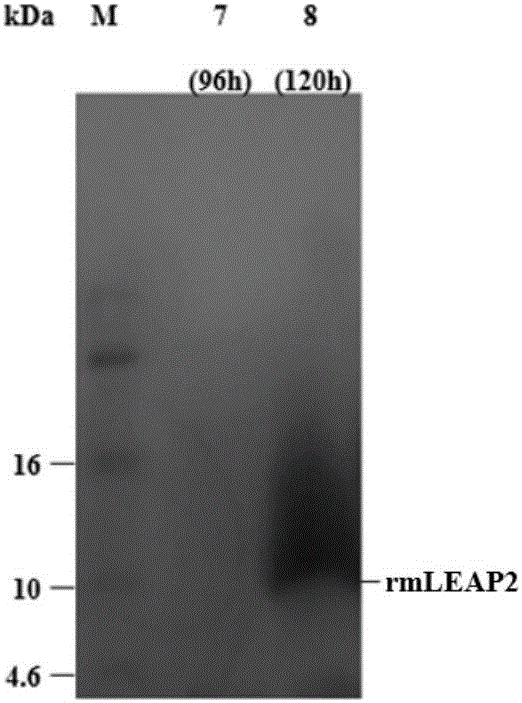
[0065] Recombinant expression and purification of channel catfish LEAP-2 mature peptide codon-optimized cDNA in Pichia pastoris X-33
[0066]2.1 Medium formula:
[0067] (BMG medium: YNB 13.4g / L, biotin 4×10 -4 g / L, potassium phosphate buffer 0.1mol / L pH6.0, glycerol 10ml / L; BMMS medium: YNB 13.4g / L, biotin 4×10 -4 g / L, methanol 7.5mL / L, potassium phosphate buffer 0.1mol / L pH6.0, sorbitol 18.2g / L).
[0068] 2.2 Optimized synthesis of cDNA of channel catfish LEAP-2 mature peptide and addition of enzyme cutting sites
[0069] Refer to Gao Bei et al. (Gao Bei, Tao Yan. Channel catfish (Ictalurus punctatus) LEAP2 mature peptide fusion expression in Escherichia coli [J]. Biotechnology Bulletin, 2014 (2): 130-135.) published channel catfish LEAP2 The cDNA sequence of the mature peptide, according to the codon preference of Pichia pastoris to the original ATG, ACA, CCC, CTT, TGG, AGA, ATC, ATG, GGT, ACT, AAA, CCC, CAT, GGG, GCA, TAC, TGT, CAG, AAT, AAC, TAT, GAG, TGT, TCC, ACG, G...
Embodiment 3
[0079] Antibacterial activity of recombinant channel catfish LEAP-2 mature peptide expressed by codon-optimized gene expression by colony counting
[0080] The selected Gram-positive bacteria were Staphylococcus aureus and Bacillus subtilis, and the Gram-negative bacteria were Escherichia coli ATCC25922. Cultivate the above-mentioned bacteria overnight in LB liquid medium, and then use fresh LB liquid medium to reduce the OD of the bacteria to 600 Adjust to 1.0, incubate at 37°C for 2 hours according to the ratio of bacterial liquid to culture supernatant 1:100, and treat the culture supernatant of the yeast transformant containing the pPICZαA empty plasmid and the bacteria in the same way as a negative control; Take 30 μL and spread it on LB solid medium, incubate at 37°C for 8 hours, and observe the number of colonies. Such as Figure 11 As shown, the culture supernatant containing the recombinant channel catfish LEAP-2 mature peptide has antibacterial activity against Sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com